AI-Powered Companion Diagnostics
Accelerate CDx Development. Empower Precision Medicine.
Indica Labs delivers a seamless, end-to-end digital pathology workflow. With our AI-powered product portfolio and custom AI development experience, we enable pharma partners to accelerate drug development with confidence.
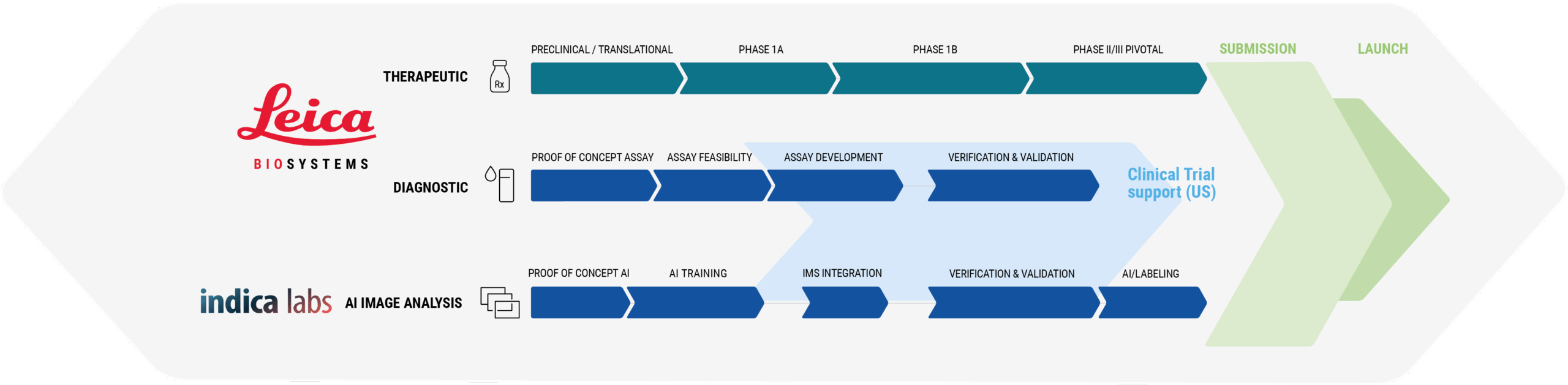
Comprehensive and Seamless CDx Co-Development
Together with our partners at Leica Biosystems, we are transforming the future of precision medicine by combining Indica Labs’ deep AI and biomarker analysis expertise with Leica Biosystems’ extensive portfolio of healthcare solutions and deep commercialization experience to deliver end-to-end companion diagnostics development.
Our Quantitative Biomarker and AI Expertise
Our team has been developing AI algorithms for oncology and immuno-oncology across a variety of imaging modalities, tissue types, and application areas since Indica Labs was founded in 2011.
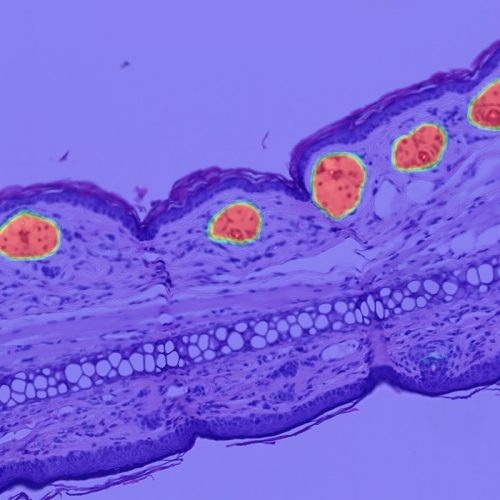
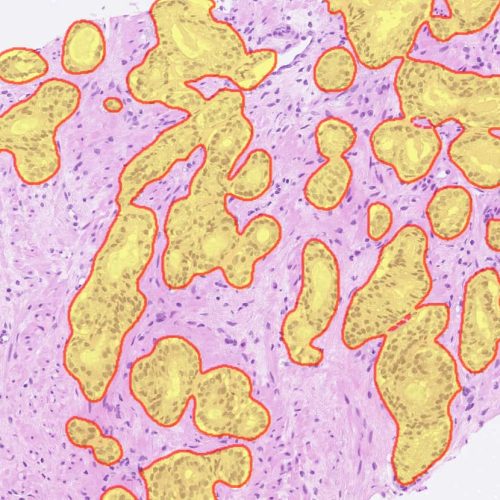
H&E
- Tumor tissue detection
- Cancer cell phenotypers
- Pan-cancer lymphocyte cell phenotyper
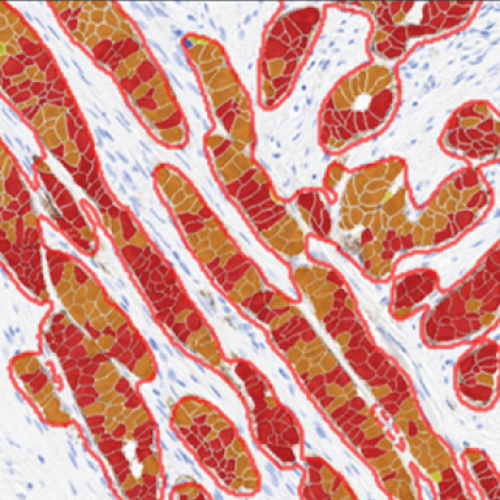
Immunohistochemistry
- Singleplex and multiplex analysis
- Antibody drug conjugate (ADC) image analysis with quantitative continuous OD scoring and bystander effect scoring
- Tumor tissue detection
- Cancer cell phenotypers
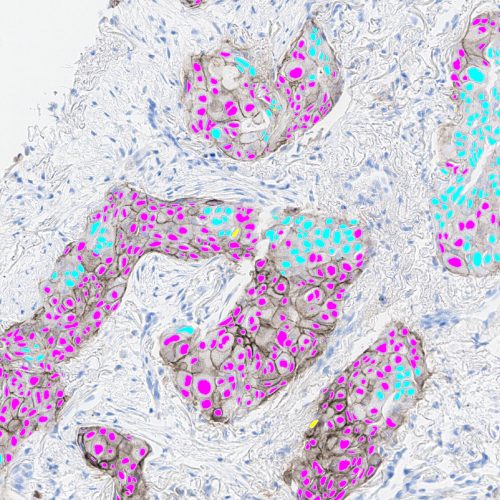
Immunofluorescence
- Antibody drug conjugate (ADC) image analysis with quantitative continuous intensity scoring and bystander effect scoring
- Nuclear, tissue, and membrane segmentation
- Cell and object phenotyping
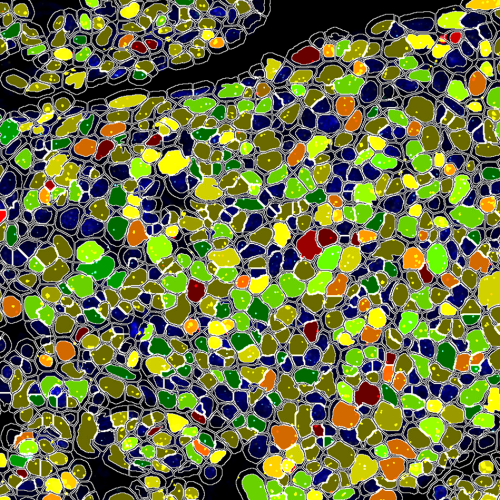
In Situ Hybridization
- Single or multi-probe chromogenic
- Single or multi-probe fluorescent
- Codetection workflows with IHC and IF
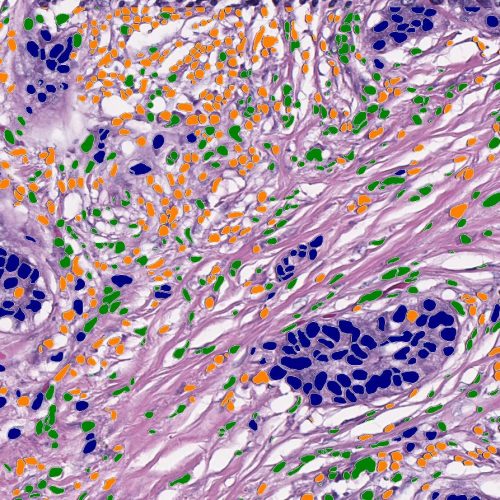
Seamless Deployment in HALO AP®
Our HALO AP® diagnostic digital pathology platforms are widely deployed throughout hospitals, reference labs, CROs, pharma, and IDNs. Companion diagnostic products can be globally deployed and easily transferred between our team, your team, and your partners, propelling your targeted therapy from discovery to commercialization.

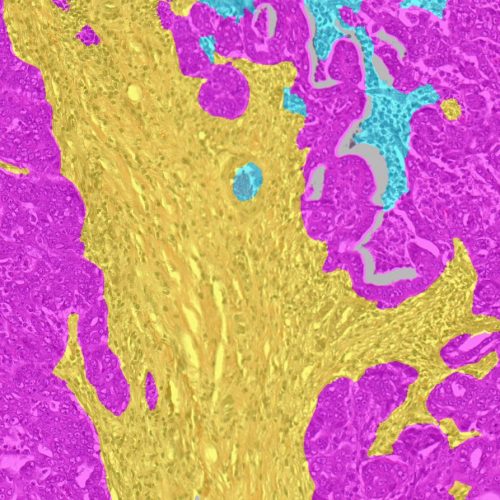
Antibody Drug Conjugate CDx
Our team leverages HALO® and HALO AI to advance ADC projects with accurate, in-depth image analysis, from tissue segmentation to advanced spatial analysis. While each workflow is unique, common components include tissue classification, nuclear and membrane segmentation, biomarker quantitative continuous scoring in the nucleus, cytoplasm, and membrane, and bystander effect evaluation.
Our analysis produces a wealth of data on ADC target expression on a cell-by-cell level with subcellular resolution, as well as rich phenotypic and spatial data on other cells in the tumor microenvironment. This data provides deep insight on ADC targets, enabling optimization of dosing strategies and validation of candidate therapies.
AI-Powered Tissue Classification
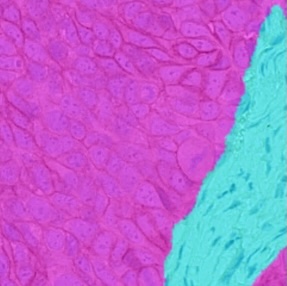
Delineate tumor/stroma or other classes of interest
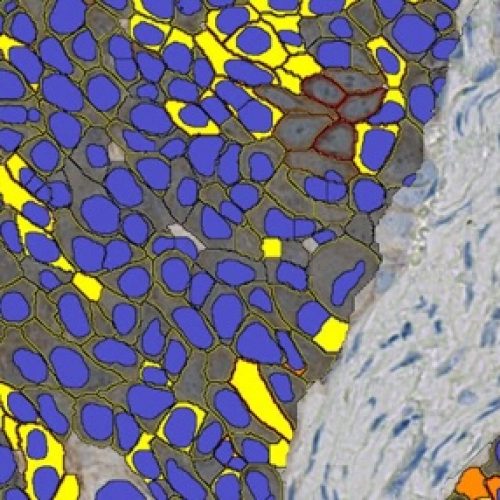
AI-Powered Subcellular Localization and QCS
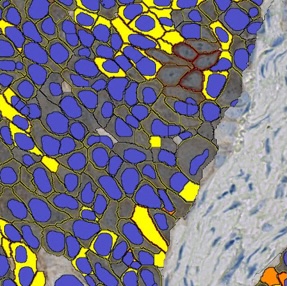
Segment cellular and membrane compartments and quantify biomarker expression
Assess Bystander Effect
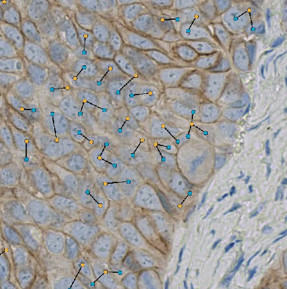
Measure Target- to Target+ distance relationships
Indica Labs Regulatory Milestones
ISO 13485 certified with a GCP-compliant Pharma Services team and a proven track record of developing and deploying products to diagnostic standards, Indica Labs has the experience and expertise to support your companion diagnostics program.
CE-IVDD HALO AP® platform
ISO 13485 certified
CE-IVDR HALO AP® platform
CE-IVDD HALO Prostate AI
510(k) cleared HALO AP Dx platform
Customers deployments of HALO Clinical AI as LDTs
HALO AP® becomes AWS Qualified Software
GCP compliant Pharma Services offering
Get in Touch
Accelerate your CDx journey by partnering with Indica Labs. Reach out today to learn more.
HALO AI Prostate is CE-marked for in-vitro diagnostic use in Europe, the UK, and Switzerland. HALO AI Prostate is For Research Use Only in the USA and is not FDA cleared for clinical diagnostic use. HALO AI Prostate is accessed via the HALO AP® enterprise digital pathology platform.
HALO AP Dx (K232833) is FDA-cleared for primary diagnostic use with the Hamamatsu NanoZoomer® S360MD Slide scanner in the USA. In addition, HALO AP Dx provides built-in compliance with FDA 21 CFR Part 11 and HIPAA.
HALO AP® is CE-IVDR marked for in-vitro diagnostic use in Europe, the UK, and Switzerland. HALO AP® is For Research Use Only in the USA and is not FDA cleared for clinical diagnostic use. In addition, HALO AP® provides built-in compliance with FDA 21 CFR Part 11, HIPAA, and GDPR.
WBS-MAR-000005v2



