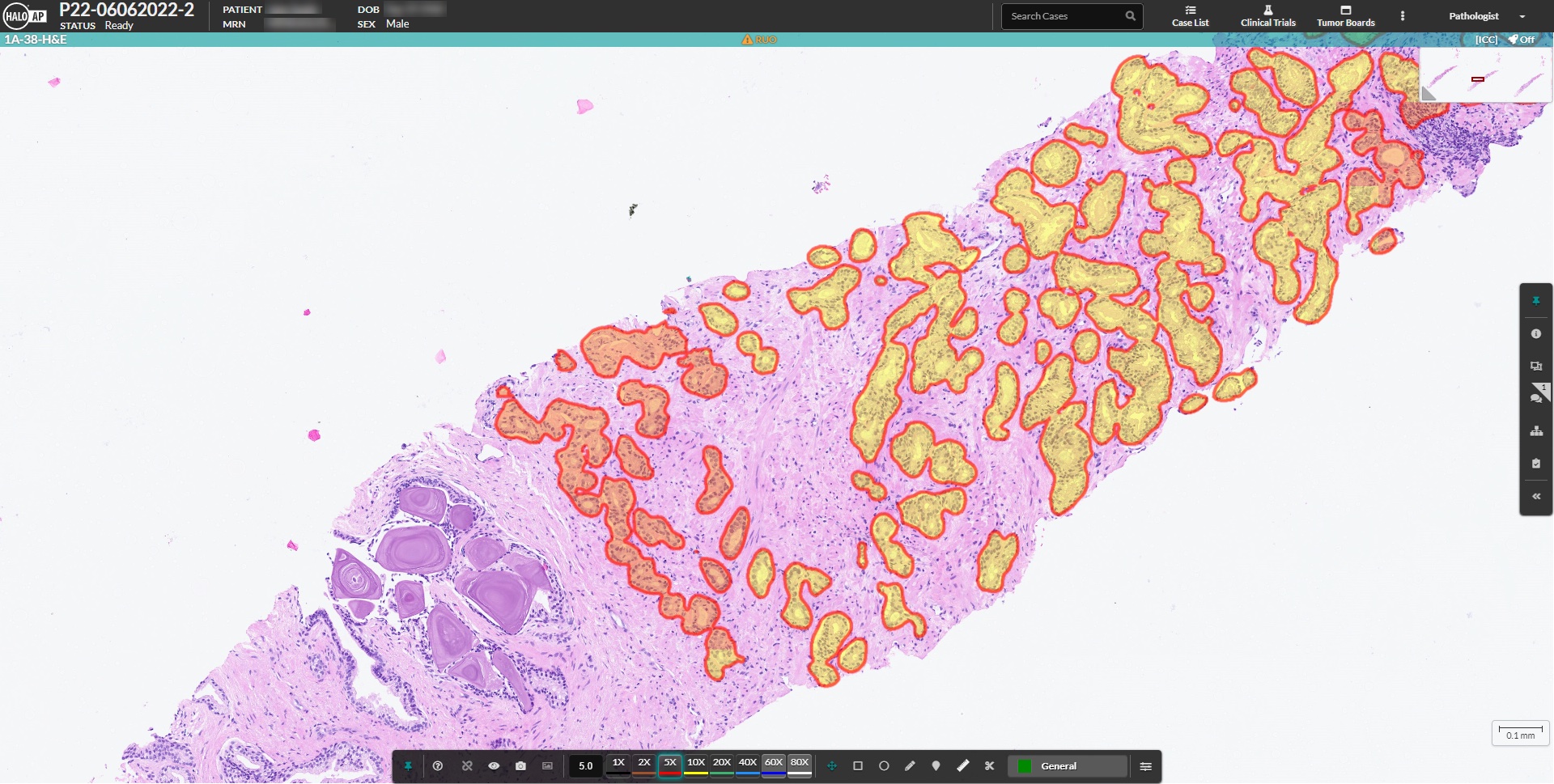
HALO Prostate AI, from Indica Labs, is a CE-IVD certified deep learning based screening tool designed to assist pathologists in the identification and grading of prostate cancer in core needle biopsies. The algorithm automatically analyzes all appropriate case slides and notifies pathologists of cases with suspected findings directly in their native workflow.

Comprehensive Analysis
The algorithm reports a comprehensive set of results and markups, including cancer detection and localization, tumor size, Gleason grading, the presence of high-grade PIN and intraductal carcinoma.
Seamlessly Integrated, AI-Enhanced Workflow
- CE-IVD marked with best-in-class clinical sensitivity and specificity
- Deploy as a screening tool to support triage or as a post-diagnostic QC-check
- Results can be shared with LIS | LIMS systems from any vendor
- Deploys in your own IT environment – cloud or on-premise – so you maintain full control of the data
- Extend your image analysis capability with further integration of IHC image analysis from Indica Labs
File Formats:
- Non-proprietary (JPG, TIF, OME.TIFF)
- Nikon (ND2)
- 3DHistech (MRXS)
- Akoya (QPTIFF, component TIFF)
- Olympus / Evident (VSI)
- Hamamatsu (NDPI, NDPIS)
- Aperio (SVS, AFI)
- Zeiss (CZI)
- Leica (SCN, LIF)
- Ventana (BIF)
- Philips (iSyntax, i2Syntax)
- KFBIO (KFB, KFBF)
- DICOM (DCM*)
*whole-slide images
HALO Prostate AI Brochure
HALO Prostate AI offers pathologists a CE-IVD certified deep learning-based tool that can reduce turnaround times while demonstrating negative predictive values of 99% or greater in validation studies.
Submit the form below to view the requested document
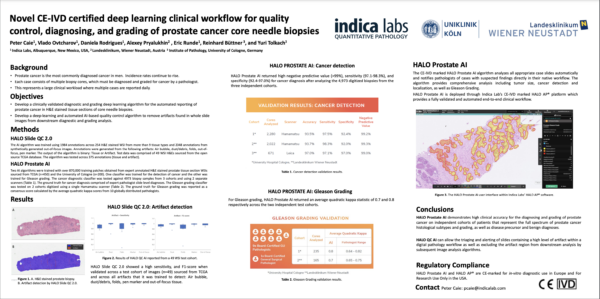
Novel CE-IVD Clinical Workflow for Analysis of Prostate Cancer Core Needle Biopsies
Learn more about Indica Labs’ novel CE-IVD certified deep learning clinical workflow for prostate cancer grading.
Submit the form below to view the requested document

HALO Clinical AI Solutions Flyer
Check out our flyer to learn more about HALO Clinical AI Solutions
Submit the form below to view the requested document

Seamless Deployment in HALO AP®
HALO Prostate AI is deployed and fully integrated into HALO AP®, the AI-powered, pathologist-driven platform for anatomic pathology workflows from Indica Labs.

Regulatory Compliance
HALO AI Prostate is CE-marked for in-vitro diagnostic use in Europe, the UK, and Switzerland. HALO AI Prostate is For Research Use Only in the USA and is not FDA cleared for clinical diagnostic use. HALO AI Prostate is accessed via the HALO AP® enterprise digital pathology platform.
HALO AP® is CE-IVDR marked for in-vitro diagnostic use in Europe, the UK, and Switzerland. HALO AP is For Research Use Only in the USA and is not FDA cleared for clinical diagnostic use. In addition, HALO AP provides built-in compliance with FDA 21 CFR Part 11, HIPAA, and GDPR.
Press Release: Indica Labs achieves CE-IVD certification for AI-based Prostate Cancer Detection and Gleason Grading Tool
Press Release
ALBUQUERQUE, NM – May 24, 2022 – Indica Labs, the leading provider of computational pathology software and services, is excited to announce a CE-IVD Mark for HALO Prostate AI, a deep learning-based screening tool designed to assist pathologists in identifying and grading prostate cancer in core needle biopsies.
Want to Learn More?
Fill out the form below to request a live demo of HALO Prostate AI or to learn more about our other clinical solutions.
You can also drop us an email at info@indicalab.com
Products & Services
Interested in purchasing or learning more about our products and services? Our highly trained application scientists are a couple of clicks away.
Software Maintenance & Support Coverage
Interested in purchasing an SMS plan? We would be happy to give you a quote.
Technical Support
Need technical support? Our IT specialists are here to help.