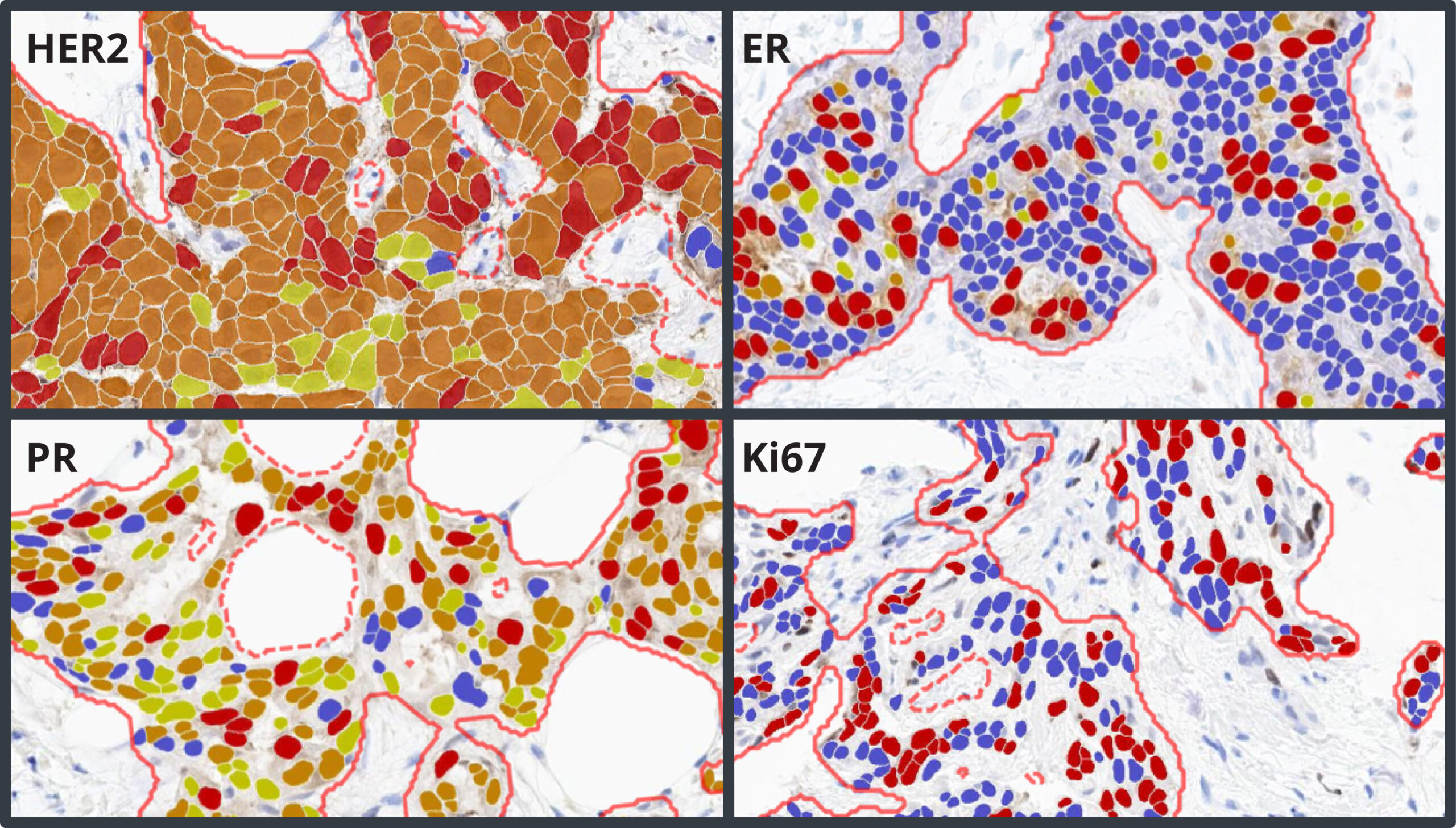
Breast IHC AI is an AI-powered image analysis tool that accurately detects regions of invasive tumor, standardizes scoring of HER2, ER, PR,and Ki67, and enables detection of low and ultralow HER2 expression in breast cancer.
Intended Use
Breast IHC AI is For Research Use Only and not intended for clinical diagnostic use.
Inputs
WSI of resections, excisions, and/or biopsies from primary invasive breast cancer
Key Outputs
- HER2: HER2 Grade: Negative, Ultralow, Low, 2, 3; HER2 Score: 0, 1+, 2+, 3+; Percent positive
- ER and PR: Intensity Score: percent of weak, moderate, and strong positive cells; AllRed Score
- Ki67: Percent of positive cells
Supported Clones
- HER2: 4B5; CB11; BMA
- ER: EP1; SP1
- PR: PgR636; Pgr A/B: 16+SAN27
- Ki67: MIB1, SP6
Breast IHC AI may be compatible with additional clones. Contact info@indicalab.com for more information.
Breast IHC AI was validated using Hamamatsu NanoZoomer S360 and Leica Aperio GT 450 scanners.

Development & Validation of an AI-based Workflow for Clinical Scoring of HER2, ER, PR, & Ki67 Immunohistochemistry in Breast Cancer Tissue
Explore Breast IHC AI, a deep learning-based workflow for evaluation of breast cancer.
File Formats
- Non-proprietary (JPG, TIF, OME. TIFF, DICOM [DCM*])
- Leica (SVS, AFI, SCN, LIF)
- Hamamatsu (NDPI, NDPIS)
- Philips (iSyntax, i2Syntax)
- 3DHistech (MRXS)
- Nikon (ND2)
- Akoya (QPTIFF, component TIFF)
- Olympus / Evident (VSI)
- Zeiss (CZI)
- Ventana (BIF)
- KFBIO (KFB, KFBF)
*whole slide images

HALO AP® and Breast IHC AI at MAPMG
Learn more about how MidAtlantic Permanente Medical Group (MAPMG) used Breast IHC AI deployed in HALO AP® to standardize prognostic scores in newly diagnosed breast cancers and increase diagnostic efficiency of first reads.

Validation and Clinical Implementation of Breast IHC AI
Learn how Kaiser Permanente deployed Breast IHC AI as a laboratory developed test.

HALO AP Integrations
Read this white paper to learn about the importance of integration and interoperability in digital pathology and explore examples of how flexible solutions like HALO AP® can enhance workflow efficiency and diagnostic accuracy.
Breast IHC AI Brochure
Learn how this AI-powered tool standardized breast cancer biomarker quantification to include HER2 low and ultralow.

Development & Validation of an AI-based Workflow for Clinical Scoring of HER2, ER, PR, & Ki67 Immunohistochemistry in Breast Cancer Tissue
Explore Breast IHC AI, a deep learning-based workflow for evaluation of breast cancer.


Indica Labs’ London HALO® User Group Meeting 2024
10 December 2024 | Indica Labs is pleased to announce our London HALO User Group Meeting to be held in London on 10 December 2024 at Hilton London Metropole from 12:00 – 16:00.

2024 Pathology Visions Pre-Conference Workshop Abstract for Indica Labs
3 November 2024 | In this one-hour pre-conference workshop, Indica Labs is pleased to host Dr. Eun Yeong Oh, MD, PhD, Assistant Regional Medical Director, Subchief of Breast Pathology, and Physician AI Champion at Mid-Atlantic Permanente Medical Group (MAPMG), who will present on the validation and implementation of a laboratory developed test using Breast IHC AI in the HALO AP® platform.

Standardize Biomarker Evaluation
Breast IHC AI standardizes biomarker scoring and reduces interobserver variability in the IHC evaluation process, without compromising accuracy.

More Efficient Workflow
Automated biomarker evaluation reduces the workload for pathologists and researchers while delivering accurate and efficient results.

Complement Your Expertise
Breast IHC AI provides consistent, standardized measurements so you are free to apply your expertise where it’s needed most, in the interpretation of results to make informed decisions.
Comprehensive Analysis
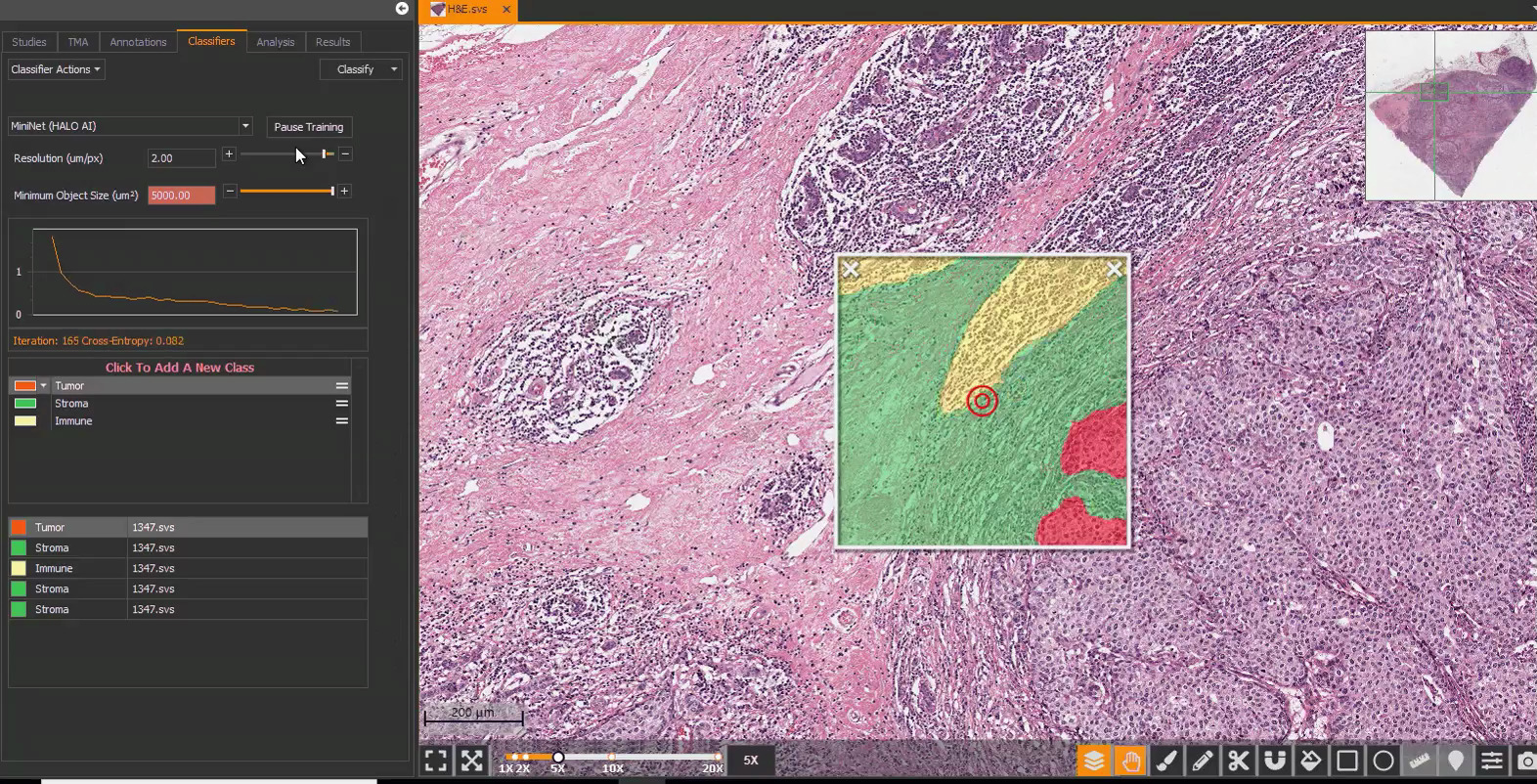
Breast IHC AI includes AI-based analysis assays for automated scoring of HER2, ER, PR, and Ki67, including analysis of HER2 low and ultralow expression. Each assay has built-in artifact exclusion, benign epithelial region exclusion, and tumor detection steps to ensure that biomarker analysis is performed accurately and consistently each and every time. Tumor cells are analyzed for expression of HER2, ER, PR, and Ki67, and a comprehensive set of results and markups are generated for each image, including clinical score and percentage positivity.

Key Output Metrics
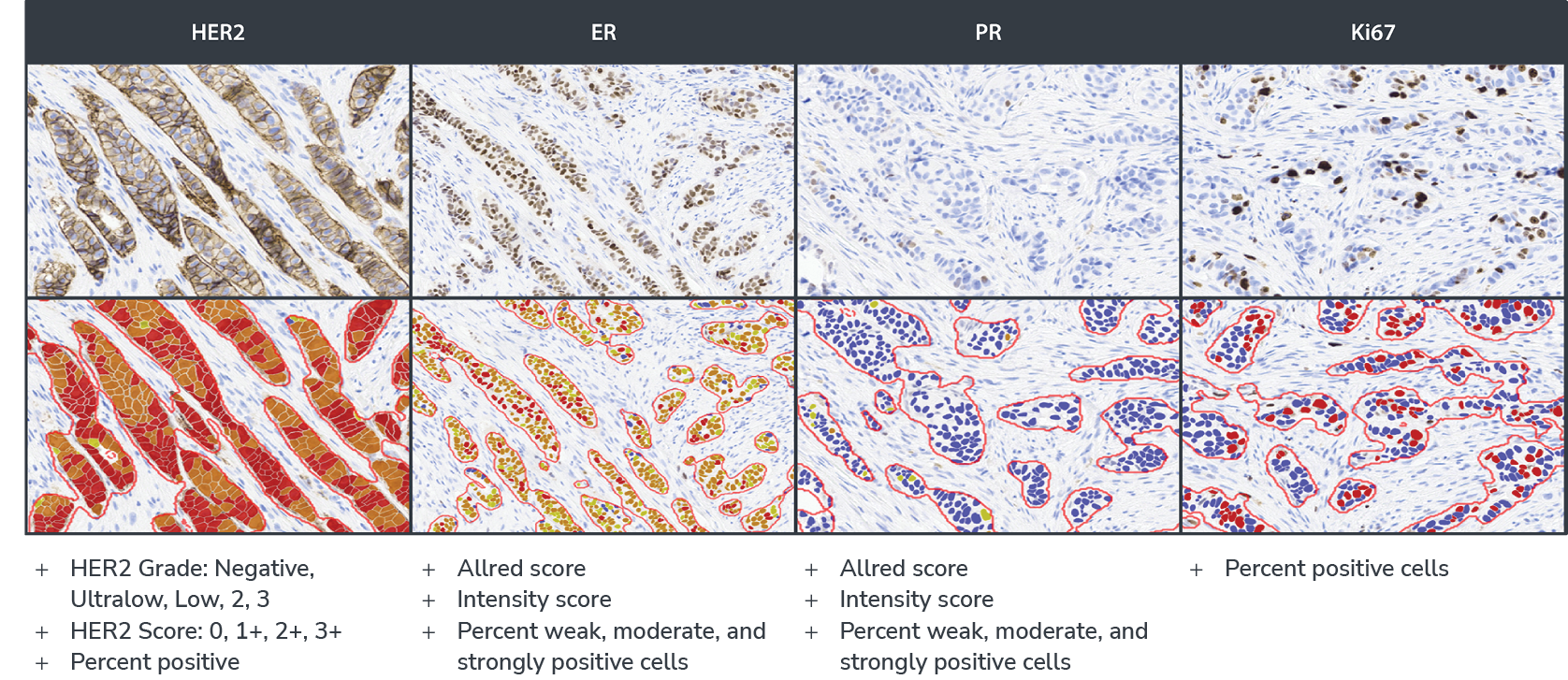
Accurately Detect HER2 Low and Ultralow Expression in Breast Cancer
The HER2 algorithm detects all levels of expression, including low and ultralow levels and outputs a Grade of Negative, Ultralow, Low, Grade 2, and Grade 3 with a corresponding Score of HER2 0, 1+, 2+, and 3+. The software assists the pathologist by automatically reporting the percentage of positive cells and biomarker scores at the slide level, along with image analysis masks which can be viewed in the HALO AP® platform.

Seamless Deployment in HALO AP®
Breast IHC AI is deployed and fully integrated into HALO AP®, the AI-powered, pathologist-driven platform for anatomic pathology workflows from Indica Labs.
Want to Learn More?
Fill out the form below to request a live demo of Breast IHC AI or to learn more about our other clinical solutions.
You can also drop us an email at info@indicalab.com
Products & Services
Interested in purchasing or learning more about our products and services? Our highly trained application scientists are a couple of clicks away.
Software Maintenance & Support Coverage
Interested in purchasing an SMS plan? We would be happy to give you a quote.
Technical Support
Need technical support? Our IT specialists are here to help.
Breast IHC AI is For Research Use Only and not intended for clinical diagnostic use. Breast IHC AI is accessed via the HALO AP® enterprise digital pathology platform.
HALO AP® is CE-IVDR marked for in-vitro diagnostic use in Europe, the UK, and Switzerland. HALO AP is For Research Use Only in the USA and is not FDA cleared for clinical diagnostic use. In addition, HALO AP provides built-in compliance with FDA 21 CFR Part 11, HIPAA, and GDPR.