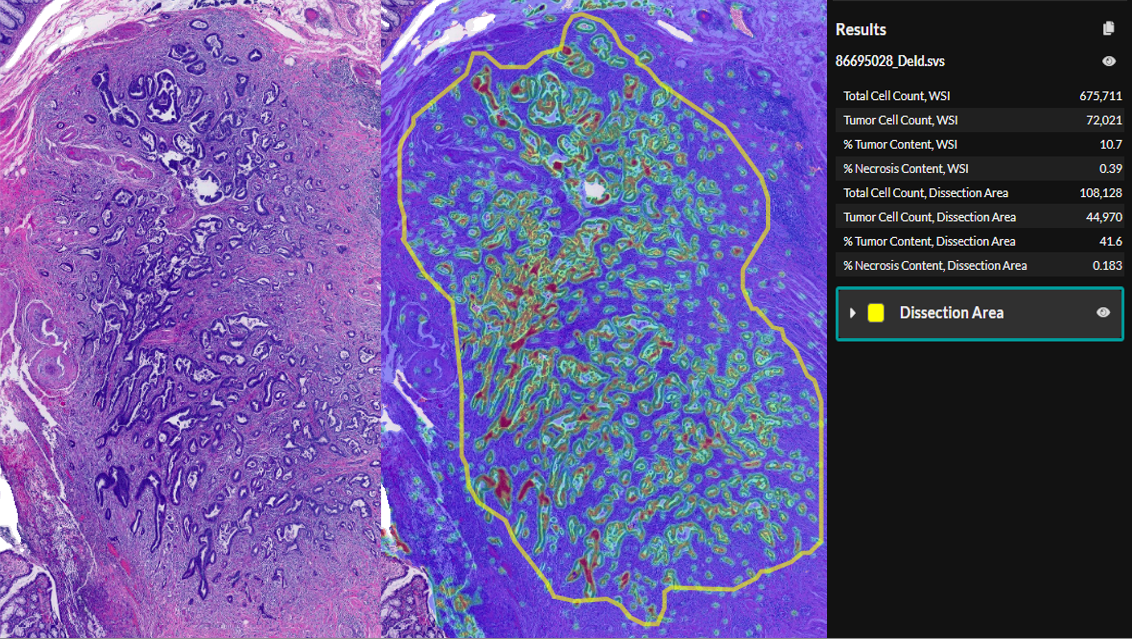
CRC Macrodissect AI and Breast HER2 AI Sneak Peek
Date: 27 March 2025
Time: 8:00 – 9:00 PDT | 11:00 – 12:00 EDT | 15:00 – 16:00 GMT
Location: Webinar
Learn about the latest advancements in AI-powered pathology
Summary
In this webinar, we’ll introduce CRC Macrodissect AI, an advanced solution designed to assist in colorectal cancer tumor content scoring and selecting regions for downstream macrodissection. CRC Macrodissect AI provides objective and standardized scoring of tumor content and enhances precision and efficiency in tissue selection. Additionally, we’ll unveil the latest advancements in HER2 analysis, with our Breast HER2 AI product now incorporating HER2 low and ultralow classifications, addressing the growing need for more precise stratification in breast cancer research.
Our speaker will walk you through the capabilities of these AI solutions, showcasing how they improve workflow efficiency, standardize results, and improve confidence. You’ll gain insights into how these applications can be utilized in real-world applications and how they integrate seamlessly into the HALO AP® enterprise digital pathology platform.
Learning Objectives
- Learn how CRC Macrodissect AI can bring efficiency and quality gains in the molecular pathology workflow
- Explore advancements to HER2 analysis in low and ultralow classifications
- Learn how AI can support clinical research
CRC Macrodissect AI is not a medical device in the EU/UK and is not intended to be used for diagnostic purposes. CRC Macrodissect AI is accessed via the HALO AP® enterprise digital pathology platform. CRC Macrodissect AI is For Research Use Only in the USA and is not FDA cleared for clinical diagnostic use.
Breast HER2 AI is For Research Use Only and not intended for clinical diagnostic use. Breast HER2 AI is accessed via the HALO AP® enterprise digital pathology platform.
HALO AP® is CE-IVDR marked for in-vitro diagnostic use in Europe, the UK, and Switzerland. HALO AP® is For Research Use Only in the USA and is not FDA cleared for clinical diagnostic use. In addition, HALO AP® provides built-in compliance with FDA 21 CFR Part 11, HIPAA, and GDPR.
Presenter

Hannah Moore
Associate Product Manager, AI Diagnostics
Indica Labs
Hannah Moore received a BEng in mechanical engineering from Lancaster University and has a background in histology. Hannah joined Indica Labs in 2023 as the Product Specialist for HALO Clinical AI Solutions. Prior to Indica Labs, Hannah worked in industry in the histology sector as a Product Manager looking after laboratory equipment and consumables. In her current role, she works closely with customers at all stages of product development to enhance the AI Diagnostics portfolio.