EZH2 is the catalytic component of Polycomb Repressive Complex 2 (PRC2) and performs trimethylation of histone H3 at lysine 27 (H3K27me3) to silence chromatin. PRC2 is known to play different roles in different cancers and inhibitors of PRC2 histone methyltransferase EZH2 are approved for certain cancers.
One aspect of this study was a mouse model to investigate KRAS-driven lung adenocarcinomas with zero, one, or two copies of Ezh2. Mice heterozygous for Ezh2 had a lower tumor burden when compared to wild-type mice. Ezh2 null mice had a higher tumor burden and shorter survival when compared to the heterozygous or wild-type mice, thus demonstrating distinct phenotypes of haplo- and full insufficiency of Ezh2.
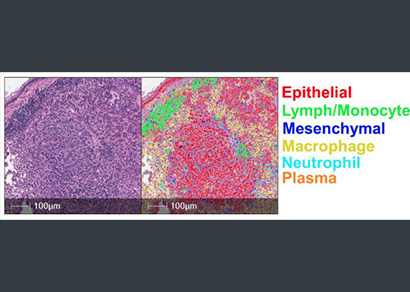
A HALO AI Object Phenotyper was trained to recognize epithelial, lymph/monocyte, mesenchymal, macrophage, neutrophil, and plasma cell types, for immune cell infiltration analysis of murine tumors on H&E images. Pathologists provided training data which included 20,491 nuclei from H&E images of 23 mouse and human tumor samples and trained the phenotyper over 400,000 iterations. The phenotyper characterized the distribution of the six cell types in tumors that were wild-type, heterozygous, or null for Ezh2. The authors found that haploinsufficiency for Ezh2 resulted in more tumor-associated lymphocytes/monocytes when compared to the Ezh2 null tumors. To confirm increased infiltration in the heterozygous tumors, the authors then performed CD3 immunohistochemistry analysis of the three genotypes of interest and found that the Ezh2 heterozygous tumors had significantly higher infiltration of CD3 compared to the wild-type and null genotypes. Together these data indicate that the heterozygous genotype is associated with an anti-tumor immune microenvironment.
The authors went on to fully characterize the Ezh2 null phenotype and found null cells were susceptible to demethylation of H3K27. In addition, they characterized the role of FOXP2, a transcription factor known to promote migration and found that loss of PRC2 leads to increased FOXP2 expression in normal and malignant lung cells. In three-dimensional cultures where FOXP2 was overexpressed, the authors found that FOXP2 overexpression had increased migratory ability. As their results in two dimensional cultures did not accurately reflect the three-dimensional biology of the spheroid studies, the authors conclude that this study not only elucidates the complex phenotypes of Ezh2 haplo- and full insufficiency but also shows the importance of investigating three-dimensional cell cultures in oncology studies.
Chan F, Byrd A, Liu J, Flight R, DuCote T, Naughton K, Song X, Edgin A, Lukyanchuk A, Dixon D, Gosser C, Esoe D, Jayswal R, Orkin S, Moseley H, Wang C, Brainson C
Nature Communications | First published 20 January 2023 |DOI https://doi.org/10.1038/s41467-023-35784-x