Digital Pathology in Clinical Trials: Driving Faster Drug Development

- By Jenna Karpel
- September 11, 2025
Overview: The Clinical Trials Module in HALO AP Platforms
The Clinical Trials module in HALO AP®, a CE-IVDR marked and AI-powered platform for primary diagnosis, and HALO AP Dx, an FDA-cleared platform for primary diagnosis, provide pathologists and researchers access to comprehensive digital pathology tools for building objective, unbiased datasets that support medical research and faster drug development. The Clinical Trials module is a cutting-edge feature within the platform, used for meticulous organization of project-specific metadata and includes an all-encompassing dashboard for easy viewing and monitoring of all projects in progress. The module also provides a blind scoring workflow that allows cases to be sent for blind review by any number of pathologists, with the option to anonymize patient data, resulting in robust, objective data analysis. The HALO AP® Clinical Trials module solves current challenges within the clinical trials process by making data more accessible, reliable, auditable, and secure.
Current Pain Points in the Clinical Trials Process
Clinical trials are conducted in phases to properly assess the safety and efficacy of a drug or medical device before it is approved and implemented for public use, ensuring the highest level of patient safety, but resulting in a clinical trial process that is lengthy and very costly. This approach can delay life-saving care for eligible patients and result in millions of dollars lost for patients and institutions, respectively. Limitations in the current clinical trial process include poor systems communications due to clinical trial data being stored in various systems and different formats leading to operational inefficiencies, inconsistencies and compromised data quality. Additionally, physical glass slides are usually shipped for analytical collaboration which introduce many logistical delays, and a non-standardized evaluation process can lead to inappropriate patient stratification, consequently leading to an increase in adverse events (AEs). These limitations make the current clinical trial process less efficient and vulnerable to error, delaying drug development and increasing risk for stakeholders.
It is possible to lessen the burden of these operational inefficiencies by utilizing a digital pathology platform like HALO AP® for clinical trial management. Digital pathology platforms are being leveraged to harmonize data, streamline collaboration, standardize biomarker analysis, ensure regulatory compliance, and offer secure, anytime-anywhere, access to the most valuable data in drug discovery.
Digital Pathology in Clinical Trials Seamlessly Integrates Data Sources
HALO AP® harmonizes data by providing a unified platform for data integration, solving a major pain point in clinical trial data management and analysis. A unified clinical trials module within an enterprise digital pathology platform consolidates all trial-related data in a structured and accessible format. The clinical trials module brings together complex datasets from multiple sources such as laboratory information systems (LIMS), electronic data capture (EDC), whole slide images (WSI), biomarker analysis, the patient’s medical history, and other metadata important for the comprehensive analysis of each patient. This workflow enhances drug discovery and clinical trials by breaking down the silos of data integration and offering a secure platform for data storage making it much easier to cross-reference clinical outcomes and perform risk-based monitoring. This architecture provides users with a comprehensive view of each patient within the trial, ensuring a human-centered approach to the clinical trial process.
The Blind Scoring Workflow: Validating Data with Confidence
The blind scoring workflow within the Clinical Trials Module of the HALO AP® platform.
The blind scoring workflow helps users achieve one of the most fundamental goals of digital pathology — to improve diagnostic accuracy by significantly reducing inter-observer variability and standardizing results.
HALO AP® enables cases within in the clinical trials module to be assigned to multiple reviewers via the blind scoring workflow, with the option to anonymize patient data, ensuring unbiased evaluation. Blind scoring allows multiple reviewers to independently score or diagnose a case without influencing one another, thus enhancing diagnostic accuracy by allowing physicians to make objective clinical decisions. Additional use cases for blind scoring include comparing scoring results from multiple pathologists and comparing pathologist scores with and without AI assistance. Additionally, HALO AP® provides users with a secure platform which can be shared with collaborators worldwide, removing logistical barriers and making global collaboration between pathologists possible. Once scoring has been completed, data can easily be exported into a single .csv file for downstream analysis. This workflow makes biomarker quantification as well as tissue and data analysis faster and more reliable, increasing the efficiency of clinical trials and drug discovery.
HALO AP® further reduces inter-observer variability through their synoptic reporting functionality, which allows users to build digital synoptic reports directly from the platform further improving the reproducibility of results across multiple reviewers. Blind scoring is also essential for AI algorithm validation, ensuring the pathologists’ review is compared to each AI output without outside influence.
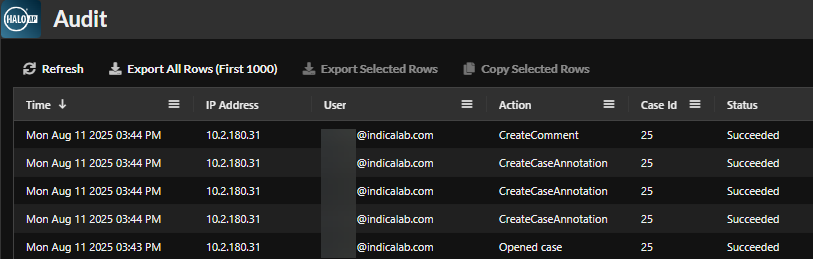
Ensuring Compliance at Every Step of the Clinical Trial Process
HALO AP® has a centralized dashboard with automated data tracking that streamlines audit-readiness and trial oversight. Historically, the audit trail of a clinical trial would have to be manually documented, scanned, and stored appropriately. HALO AP® eliminates these inefficiencies with a comprehensive automated audit log that records exactly who accessed each slide, what was viewed or annotated, and what changes were made. This automated, digitally time-stamped workflow fulfills regulatory requirements and establishes compliance. The platform also provides additional security measures for electronic signatures like multi-factor authentication (MFAs), eliminating the days of an ink signature on paper and satisfying the strict requirements across regulatory bodies. Bulk, automatic, and secure de-identification of protected health information (PHI) is also a feature available in the HALO AP® platform. This action can be executed at the time the user assigns blind scoring to reviewers or before data is exported for analysis. Either way, this workflow ensures patient confidentiality requirements under many data protection laws including HIPAA and GDPR.
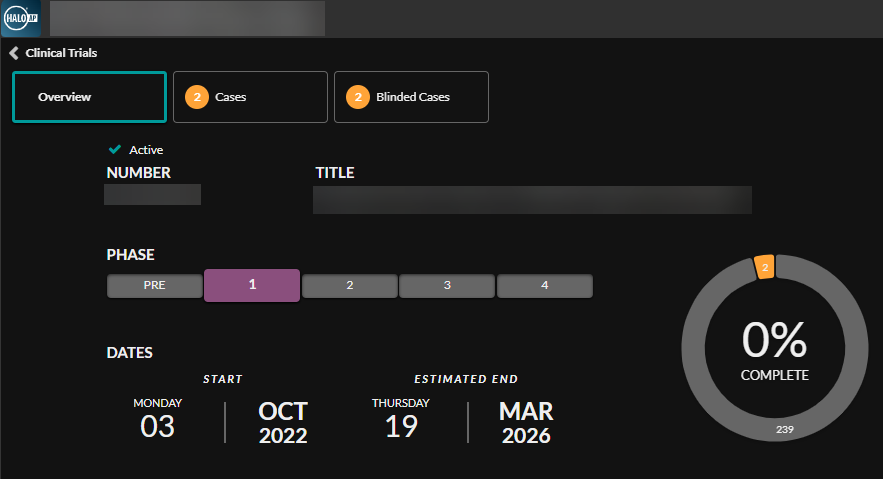
A Real-Time Comprehensive Dashboard with Visibility of Trial Metrics: Managing Costs and Improving Patient Care
Real-time dashboards and progress indicators like the ones seen in the HALO AP® and HALO AP Dx Clinical Trials module, can be utilized by clinical trials researchers, stakeholders, and regulatory affairs professionals to gain real-time visibility into the progress of a clinical trial. The dashboard displays the overall progress of the study while the indicator bar shows the progress of the individual parent case as well as the progress of any associated blind scoring cases. This operational visibility allows users to identify any inefficiencies that could benefit from early intervention, protecting the care of patients and trial assets at each step of the clinical trial process.
In addition to benefiting all stakeholders in clinical trials, any gains in operational efficiency also benefit pharma by reducing clinical trial costs. Finally, having a centralized viewing dashboard helps monitor adverse events and allows easy risk-based monitoring which is essential for patient care and trial viability.
The clinical trials module in HALO AP® is advancing the phase-based process by providing a secure, intuitive, and unified platform for centralized and remote access to trial data. This benefits each and every patient and caregiver by significantly enhancing the efficiency of drug discovery thereby getting patients the care they need— quicker.
To learn more or to try out HALO AP® or HALO AP Dx for yourself, schedule a free demo or reach out to us at info@indicalab.com!
HALO AP Dx (K232833) is FDA-cleared for primary diagnostic use with the Hamamatsu NanoZoomer® S360MD Slide scanner in the USA. In addition, HALO AP Dx provides built-in compliance with FDA 21 CFR Part 11 and HIPAA.
HALO AP® is CE-IVDR marked for in-vitro diagnostic use in Europe, the UK, and Switzerland. HALO AP® is For Research Use Only in the USA and is not FDA cleared for clinical diagnostic use. In addition, HALO AP® provides built-in compliance with FDA 21 CFR Part 11, HIPAA, and GDPR.
WBS-MAR-000006v1