Indica Labs and Lunit Announce a Strategic Alliance for Seamless Integrated Digital Pathology AI Workflows

Enabling clinical effectiveness via a fully interoperable and integrated digital pathology solution.
Albuquerque, NM and Seoul, South Korea – 25 April 2023 – Indica Labs, an industry leader in quantitative digital pathology and image management innovations, and Lunit, a leading medical AI software platform company focused on cancer biomarkers announced an agreement to provide a fully interoperable solution between Indica Labs’ HALO AP® image management software platform and Lunit’s suite of AI pathology products. The combined solution is already in use at Guardant Health, a leading precision oncology company.
“Leveraging the power of digital pathology and artificial intelligence to improve tissue-based research and patient care is at the core of our mission,” said Steven Hashagen, founder and CEO of Indica Labs. “This valued collaboration enables us to seamlessly integrate Lunit’s AI pathology solutions, including Lunit SCOPE PD-L1 for non-small cell lung cancer (NSCLC) into HALO AP, our CE-IVD certified clinical image management platform.”
Brandon Suh, CEO of Lunit said, “The partnership with Indica Labs, a market leader in digital pathology, with interoperable workflow integration, will enable pathologists and labs to deliver faster, more accurate, and more reproducible answers using Lunit’s AI solutions.”
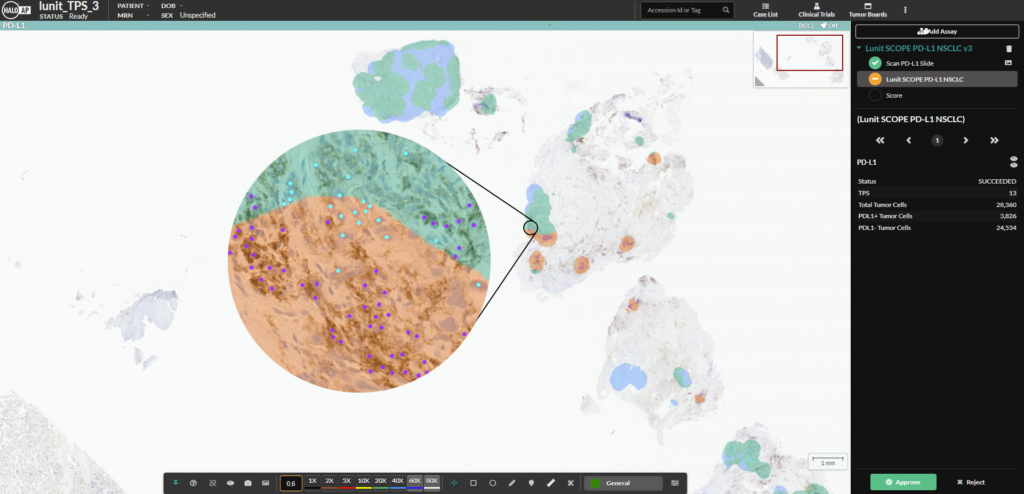
An example of the Lunit SCOPE PD-L1 NSCLC markup image in HALO AP. The cyan, blue and orange masks reflect the local tumor proportion score (TPS). Positive cells are shown in purple while negative cells are shown in cyan. The overall TPS is reported at the right.
Lunit SCOPE PD-L1 NSCLC is a CE-marked AI solution for detecting and analyzing PD-L1 TPS, a cancer biomarker. The solution may assist pathologists by minimizing interpretation variability and allowing better prediction of treatment outcomes for NSCLC patients, as demonstrated in study findings published in the European Journal of Cancer.
Indica Labs developed the HALO AP platform specifically to meet the needs of anatomic pathology labs worldwide. The CE-IVD certified HALO AP platform supports a wide range of tissue-based workflows, including primary diagnosis, secondary consults, clinical trials, synoptic reporting, quantitative analytics and AI. One of the key advantages of HALO AP is the seamless integration of AI solutions into the workflow, whether developed by Indica Labs or third parties. To simplify integration, a HALO AP software development kit (SDK) is available to all algorithm developers. With HALO AP, users can utilize algorithms in an automated, stepwise workflow that can be locked, secured and audited to prevent tampering or misuse.
Lunit SCOPE PD-L1 NSCLC can be activated in the HALO AP user interface. Analysis progress, all results and visual markup images can be reviewed in HALO AP, so users never have to leave the viewer or shift between systems. With HALO AP’s built-in workflow management tools, an algorithm can automatically run upon slide intake so that the results are ready for the pathologist to review the case. By integrating Lunit’s powerful AI solutions into HALO AP, pathologists and researchers can benefit from the combined capabilities of both platforms, providing them with advanced tools to help them make more informed decisions.
About Indica Labs:
Indica Labs is the world’s leading provider of computational pathology software and image analysis services. Our flagship HALO® and HALO AI platform facilitates quantitative evaluation of digital pathology images. HALO Link facilitates research-focused image management and collaboration while HALO AP® enables collaborative clinical case review. HALO AP is CE marked in Europe and has not been cleared or approved by the US Food and Drug Administration (FDA) and is intended for Research Use Only. Through a combination of precision, performance, scalability, and usability our software solutions enable pharmaceutical companies, diagnostic labs, research organizations, and Indica’s own contract pharma services team to advance tissue-based research, clinical trials, and diagnostics.
About Lunit:
With AI, Lunit aims to ‘conquer cancer,’ one of the leading causes of death worldwide. Lunit is an AI software company devoted to developing AI solutions for precision diagnostics and therapeutics, to find the right diagnosis at the right cost, and the right treatment for the right patients. Lunit, a portmanteau of ‘learning unit,’ is a deep learning-based medical AI company devoted to developing advanced medical image analytics and data-driven imaging biomarkers via cutting-edge technology.
Founded in 2013, Lunit has been acknowledged around the world for its advanced, state-of-the-art technology and its application in medical images. Its technology has been recognized at international AI competitions surpassing top companies like Google, IBM, and Microsoft. As a medical AI company with a focus on clinical evidence, the company’s findings are presented in major peer-reviewed journals such as the Journal of Clinical Oncology and JAMA Network Open, and global conferences including ASCO and AACR.
After receiving FDA clearance and the CE Mark, Lunit INSIGHT CXR and MMG are clinically used in approximately 2,000 hospitals and medical institutions across more than 40 countries. Lunit SCOPE PD-L1 is CE marked in Europe and has not been cleared or approved by the US Food and Drug Administration (FDA) and is intended for Research Use Only. Lunit is headquartered in Seoul, South Korea with offices and representatives around the world.
Media contacts:
Jussarang Lee
oncology@lunit.io
https://www.lunit.io/
Kate Lillard, PhD
kate@indicalab.com