The Enterprise Platform Powering Diagnostic Pathology
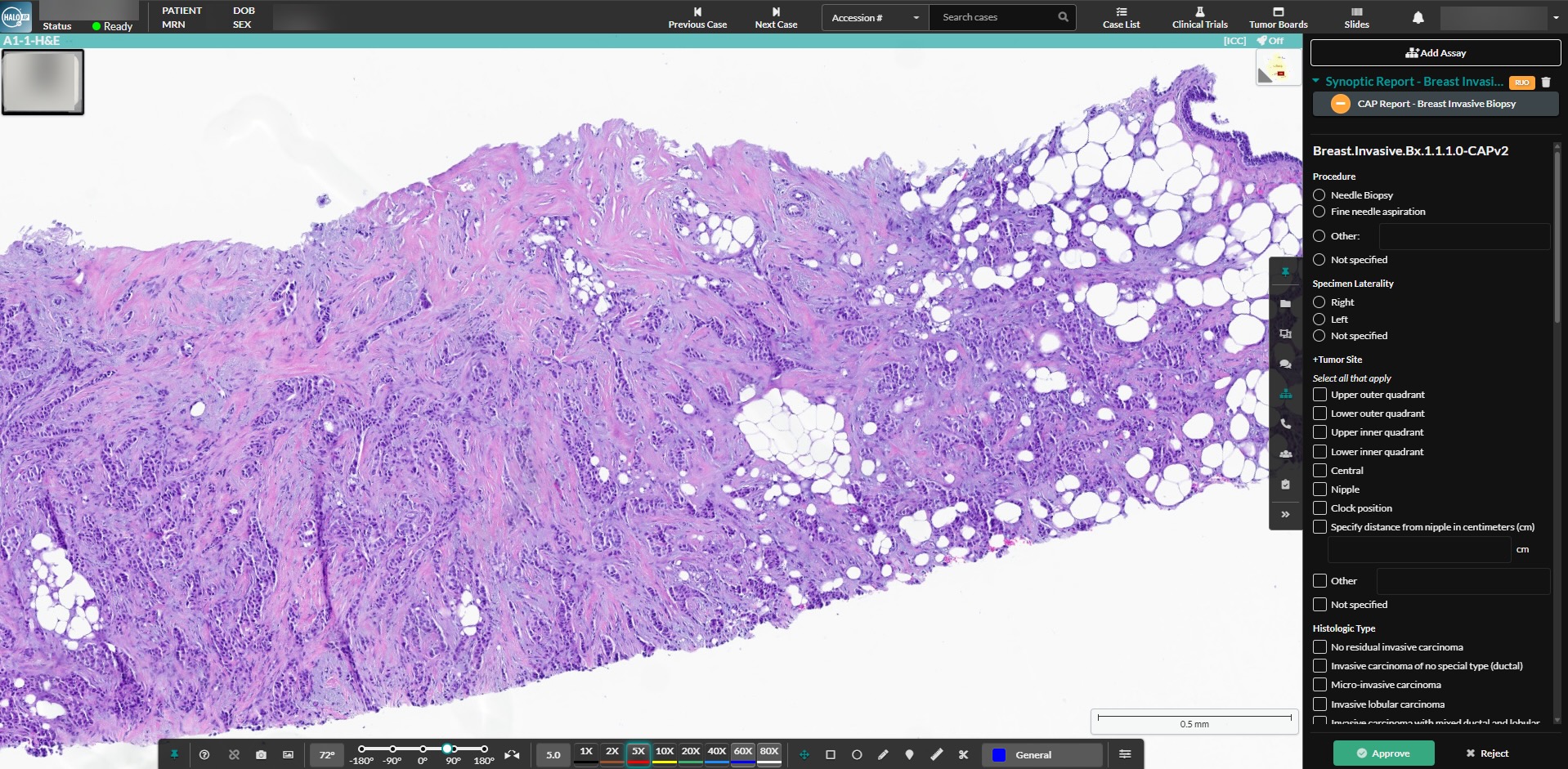
HALO AP Dx is a case-centric pathology platform that enables primary diagnosis, remote working, and other routine workflows in anatomic pathology. For primary diagnostic applications, HALO AP Dx is FDA cleared in the US, while HALO AP® is CE-IVDR marked in the EU, Switzerland, and the UK. Learn from a practicing pathologist how HALO AP® improved patient care in their lab.
Accuracy in Diagnosis, Efficiency in Care
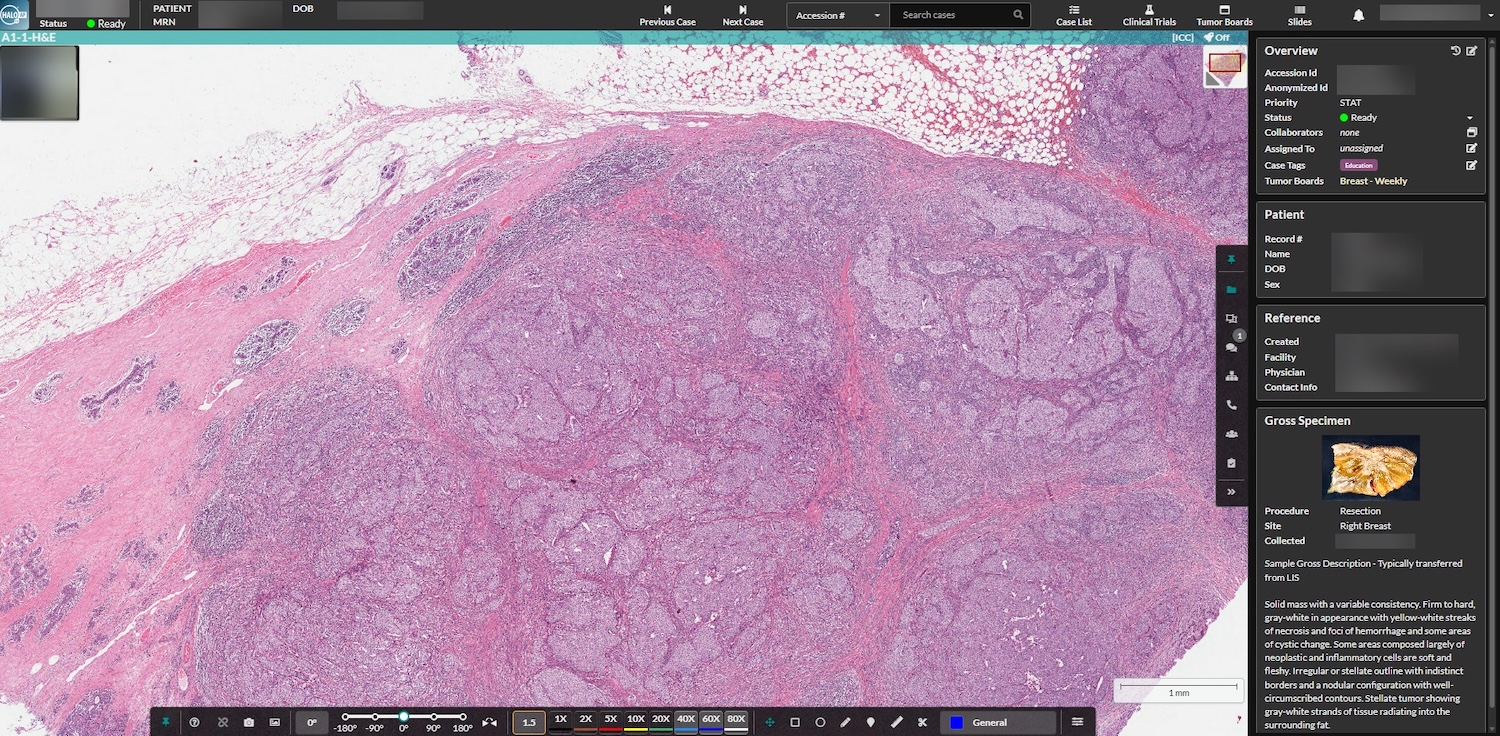
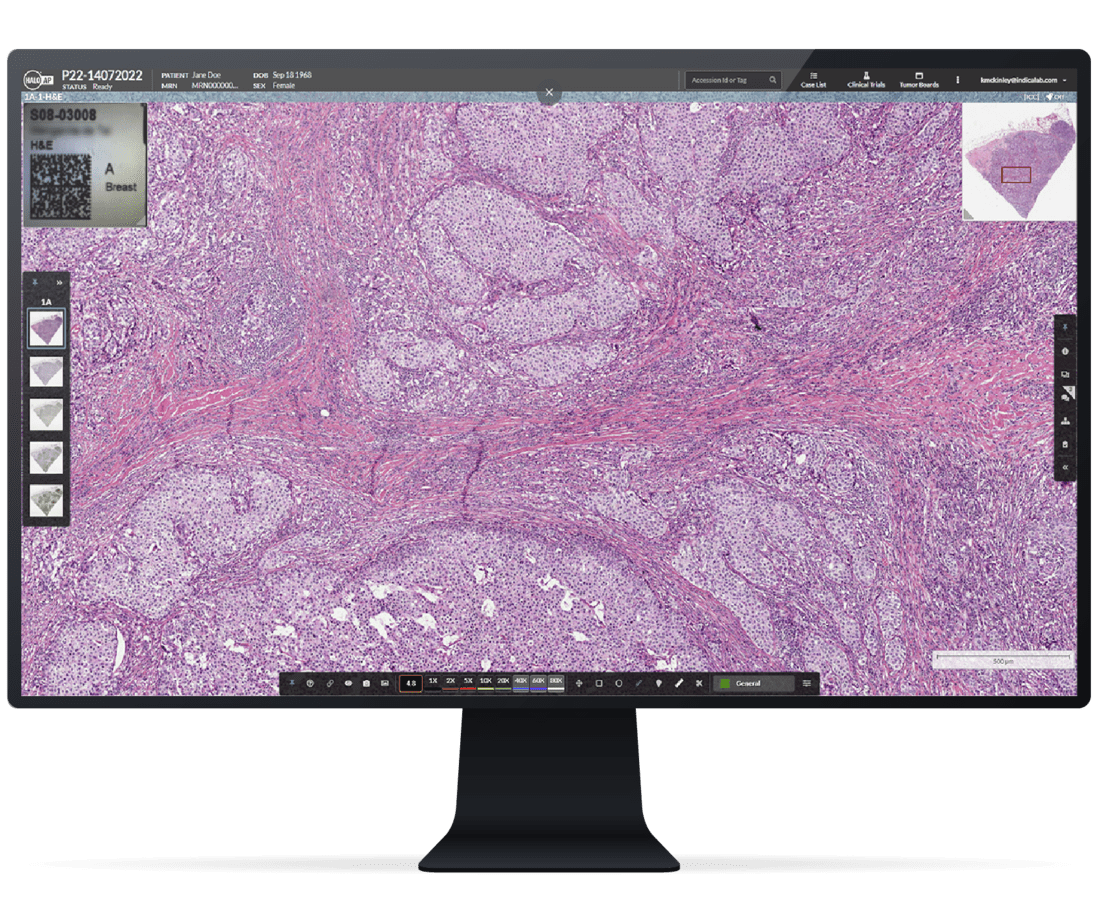
The case-centric HALO AP Dx platform lets pathologists review cases remotely the moment they’re scanned, delivering faster diagnoses without sacrificing accuracy.


Collaborate Anytime, Anywhere
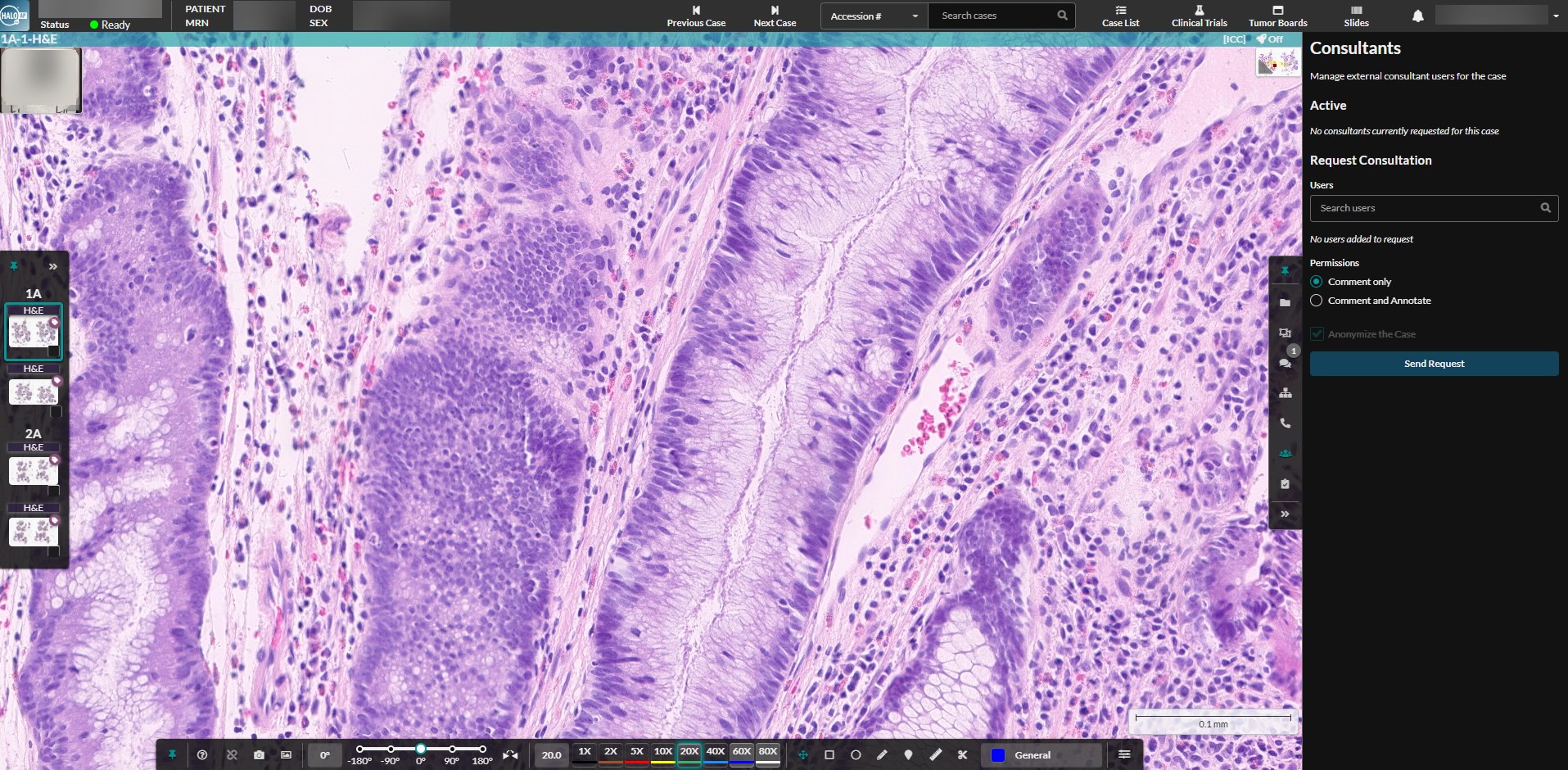
Users can collaborate remotely with colleagues internal or external to their institution. Built with unmatched security, the platform enables anonymized case sharing and real-time case conferencing for external consultations, collaborative reviews, or medical education training.
HALO AP Dx enables the highest quality of patient care by offering a unified platform for anytime, anywhere collaboration.

Customized and Secure Deployments
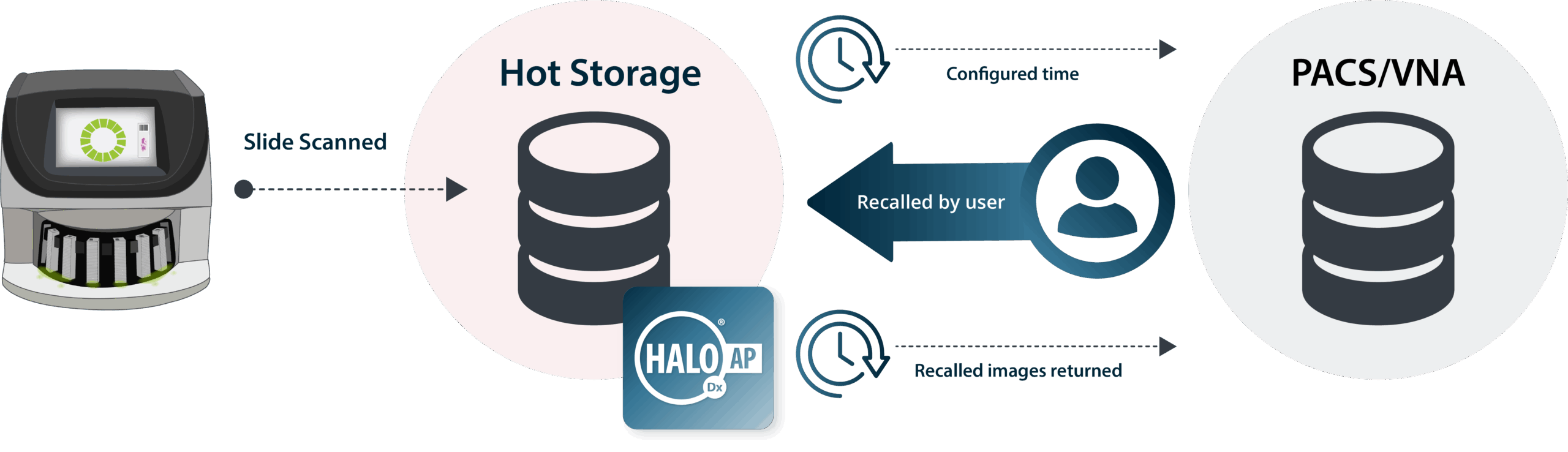
HALO AP Dx gives you the flexibility to deploy on-premise or in the cloud, seamlessly fitting into your IT framework. With enterprise-grade administration, scaling to support your growing lab is simple and secure.
Our Cloud Services team ensures seamless migration and ongoing optimization of your cloud environment, delivering performance, flexibility, and security you can rely on. You can count on our AWS Certified Solution Architects and expert technical support.

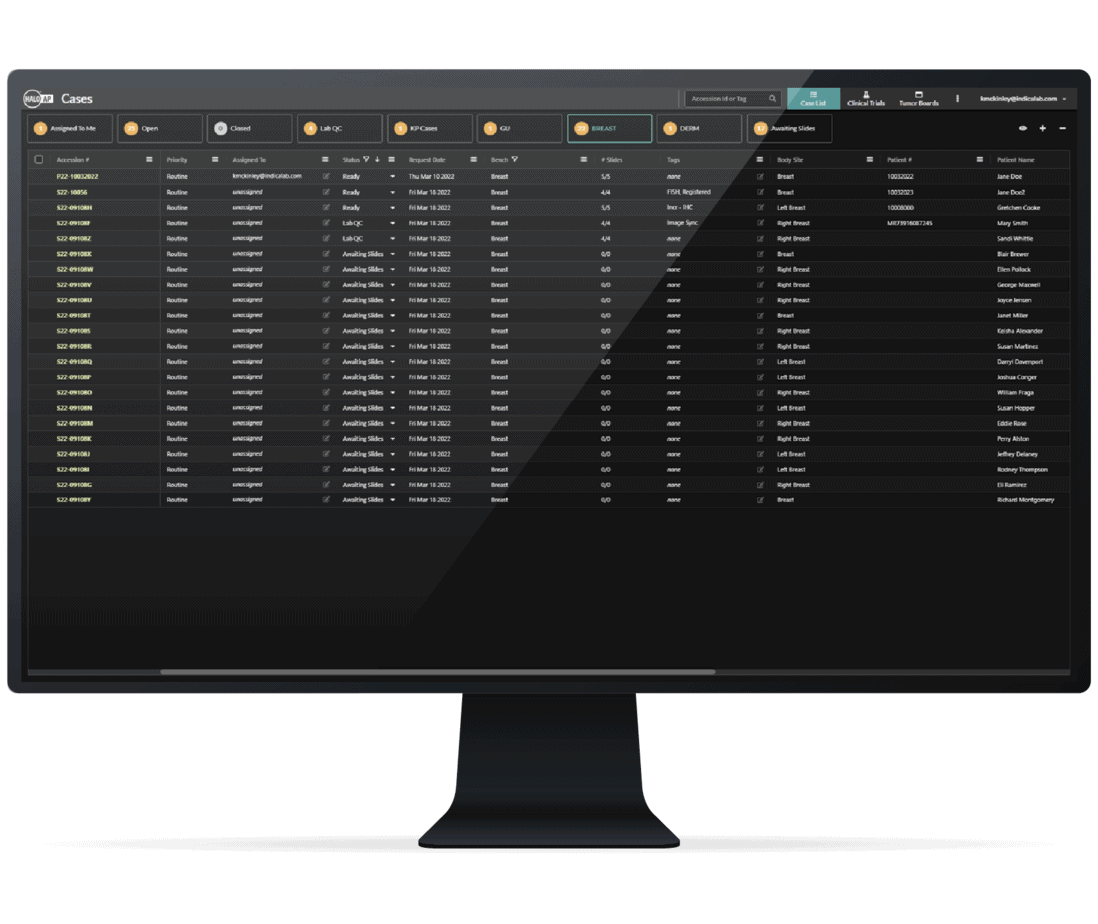
HALO AP Dx Integrates into Existing Laboratory Frameworks

Step 1
Step 2
Step 3
Step 4

Step 5

Step 6
HALO AP Dx integrates with your LIS or LIMS so that pathologists can focus on patient diagnostics. By delivering the right data at the right time within a single platform, it streamlines workflows and eliminates unnecessary steps. With open, API-first connectivity, you gain seamless access to the information you need to work faster and more efficiently.
Interested in learning more?
Schedule a call to see how HALO AP Dx can meet your needs.
Comprehensive Integration, Training, and Support Services
At Indica Labs, we’re committed to delivering exceptional support every step of the way. Our applications team provides hands-on training to ensure your staff is confident from day one, while our technical experts partner with your IT department to streamline deployment.
Already using an image management system? We know that transitioning can feel daunting, but with our proven process, it’s easier than you think. Connect with us to discover how seamless the switch can be.

Platform Compatibility
HALO AP Dx is approved for use with the Aperio GT 450 DX Scanner and Dell U3223QE display for SVS and DICOM file formats. With the Barco MDPC-8127 display, HALO AP Dx is approved for use with SVS file format.
HALO AP Dx is also approved for use with the Hamamatsu NanoZoomer S360MD slide scanner with Barco MDPC-8127 display for NDPI image format.
Please email us for information on existing LIS | LIMS integrations.
FDA-Cleared File Formats:
- Leica Biosystems (SVS and DICOM)
- Hamamatsu (NDPI)
Devices:
- Windows PC
- Macintosh
Browsers:
- Chrome
- Microsoft Edge

Looking to incorporate AI and image analysis?
Learn about our HALO AP® platform that seamlessly integrates image analysis, AI, and third-party AI in a CE-IVDR marked platform.

Features of the HALO AP Dx Diagnostic Digital Pathology Platform
HALO AP Dx makes remote collaboration simple. Built-in case conferencing supports real-time, multi-user diagnostic discussions, while external consultation tools make it easy to share cases securely with outside experts for second opinions. By unifying these capabilities within a single platform, HALO AP Dx streamlines communication, strengthens diagnostic confidence, and ultimately enhances the quality of patient care.

Streamline tumor board preparation by assigning cases, slides, and annotated regions in just a few clicks. Instantly access and navigate to regions of interest and easily add case notes to keep discussions focused and efficient.
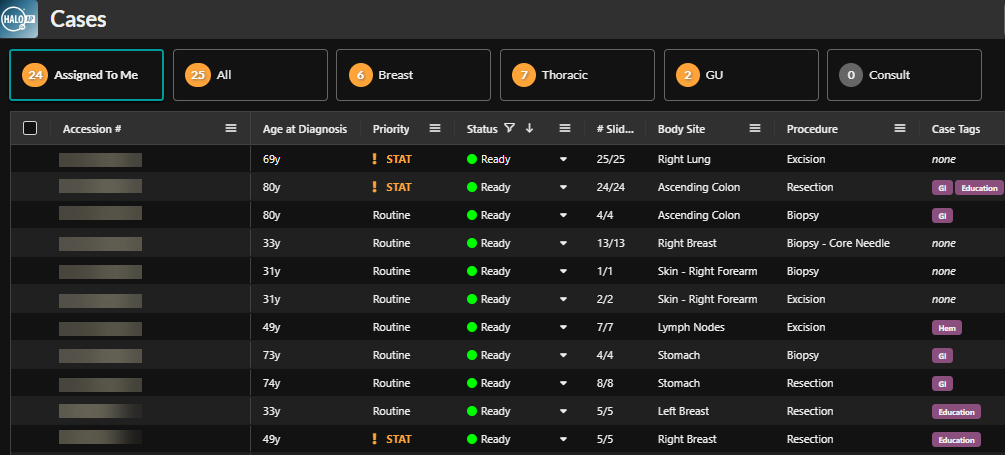
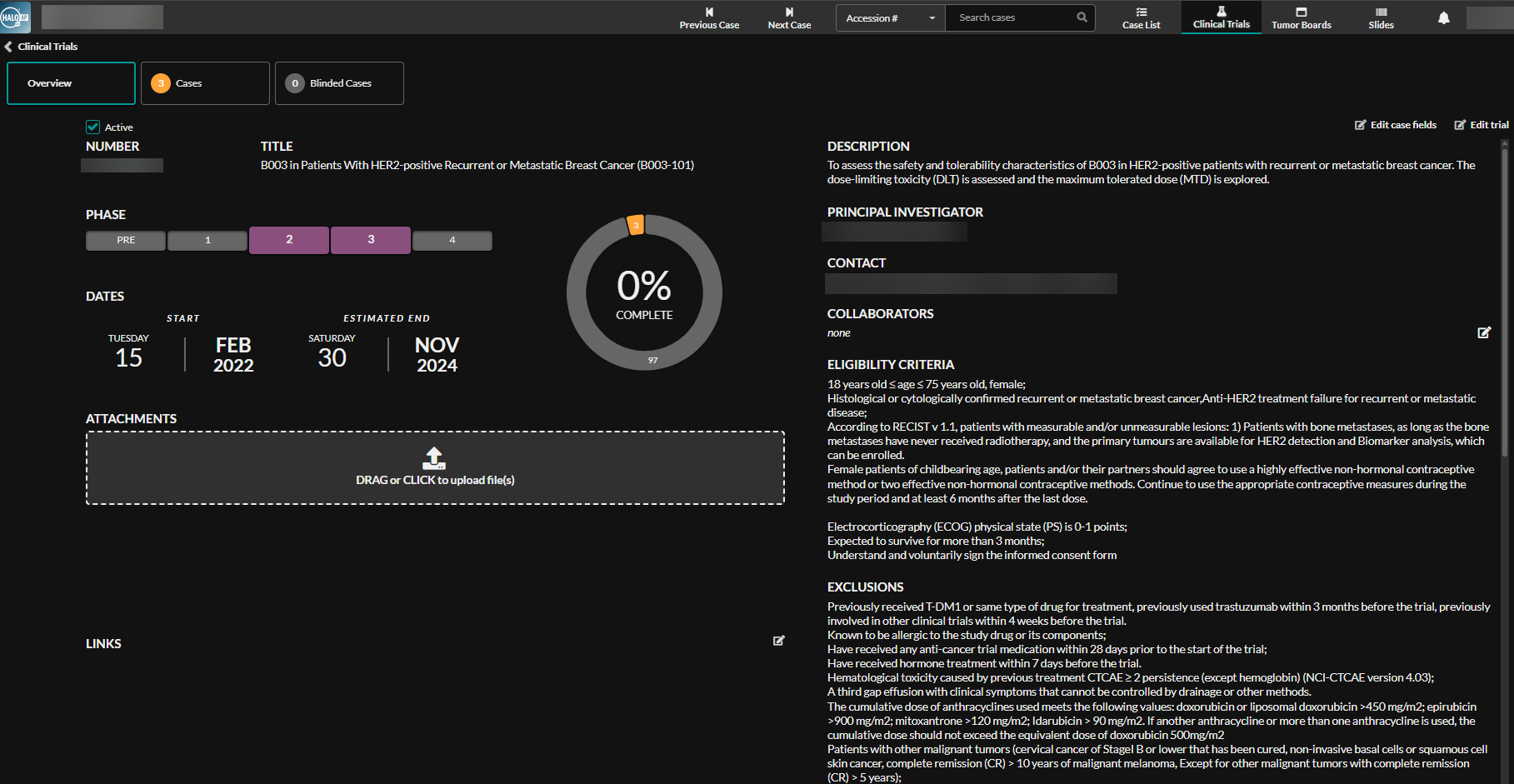
HALO AP Dx streamlines clinical trial management* across hospitals and CROs through a single, intuitive interface. Coordinators can efficiently create, manage, and monitor trials, while the blind scoring feature simplifies blinded workflows, making clinical trials and research studies easier to conduct.
*Not intended for clinical diagnostic purposes
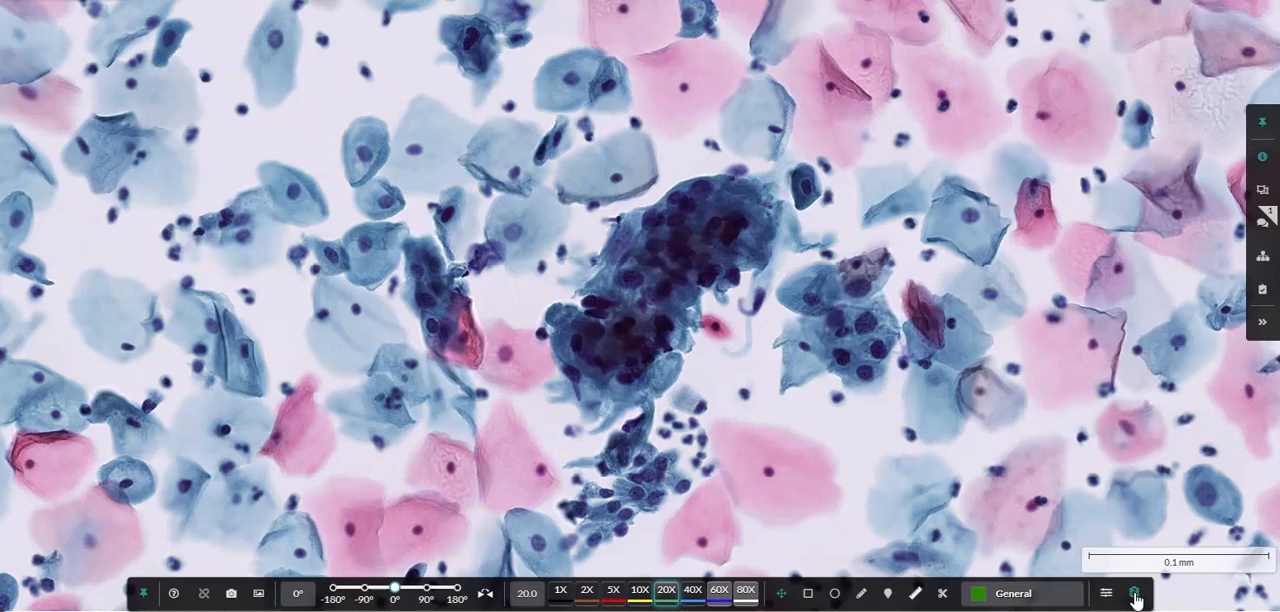
HALO AP Dx enables advanced z-stack viewing across key pathology specialties, including dermatopathology, hematopathology*, and cytology*.
*Frozen sections, cytology, or non-FFPE hematopathology specimens are for Research Use Only
Knowledge Center
Learn more about HALO AP Dx by exploring the tabs below.

HALO AP® and HALO AP Dx Brochure
Download our brochure to learn more about how HALO AP®, the CE-IVDR-cleared image management system and HALO AP Dx, FDA 510(k)-cleared for primary diagnosis, are deployed to improve clinical workflows

Indica Labs Reports 260% Clinical Revenue Growth and Expansion into New Global Markets
Albuquerque, NM, December 17, 2025 – Indica Labs, the global leader in AI-powered digital pathology, announced today that clinical revenue growth rate for HALO AP®

Indica Labs Receives FDA Clearance for Enterprise Digital Pathology Platform with Industry Leading DICOM-Compatible Aperio Scanner from Leica Biosystems
ALBUQUERQUE, NM, and VISTA, CA, December 3, 2025 – Indica Labs, the global leader of AI-powered digital pathology, and Leica Biosystems, a global leader in

Indica Labs Receives FDA Clearance for HALO AP Dx Digital Pathology Platform for Use with Hamamatsu Images Acquired with the NanoZoomer® S360MD Slide Scanner
Albuquerque, NM, and Hamamatsu City, Japan – 22 May, 2024 – Indica Labs, an industry leader in AI-powered digital pathology solutions, and Hamamatsu Photonics K.K.,

Indica Labs Receives First FDA Clearance for HALO AP Dx Digital Pathology Platform
Albuquerque, NM – 8 May 2024 – Indica Labs, the leading provider of digital pathology solutions, announced today that it received FDA clearance for HALO

Introducing HALO AP Dx: An Enterprise Pathology Platform for Primary Diagnosis
13 June 2024 | Join us for this 1-hour webinar to see a live demonstration of HALO AP Dx, the 510k-cleared digital pathology platform from

Looking Back: Reviewing 2025 at Indica Labs
As we reach the end of 2025, the Indica Labs team extends our sincere thanks to the customers and collaborators who helped make this year
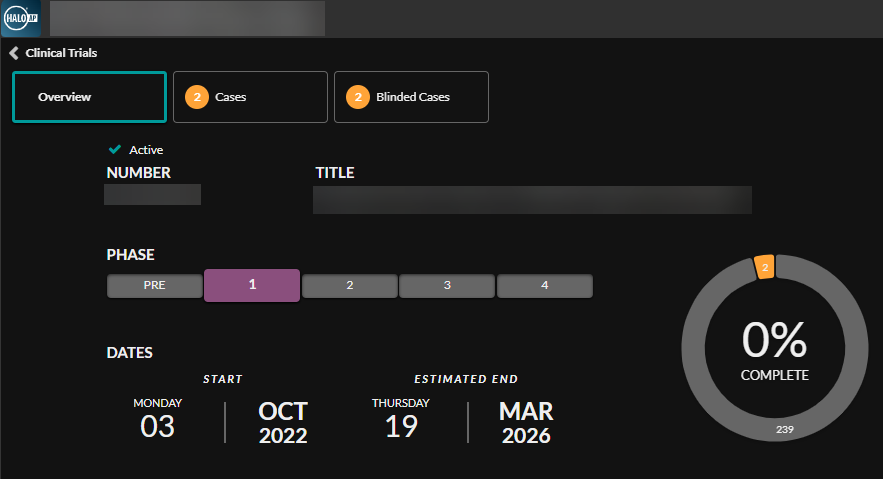
Digital Pathology in Clinical Trials: Driving Faster Drug Development
The Clinical Trials module in HALO AP® and HALO AP Dx provides pathologists and researchers access to comprehensive digital pathology tools for building objective, unbiased

Looking Back: Reviewing 2024 at Indica Labs
As 2024 draws to a close, we at Indica Labs would like to thank the customers and collaborators who helped fill 2024 with exciting discoveries,

Enhancing Patient Care with Seamless Clinical Collaboration: Streamlining Tumor Boards and MDTs with the Tumor Board Functionality of HALO AP® and HALO AP Dx
With the HALO AP platforms and their dedicated Tumor Board functionalities, pathologists can enhance multidisciplinary communication and improve treatment planning.
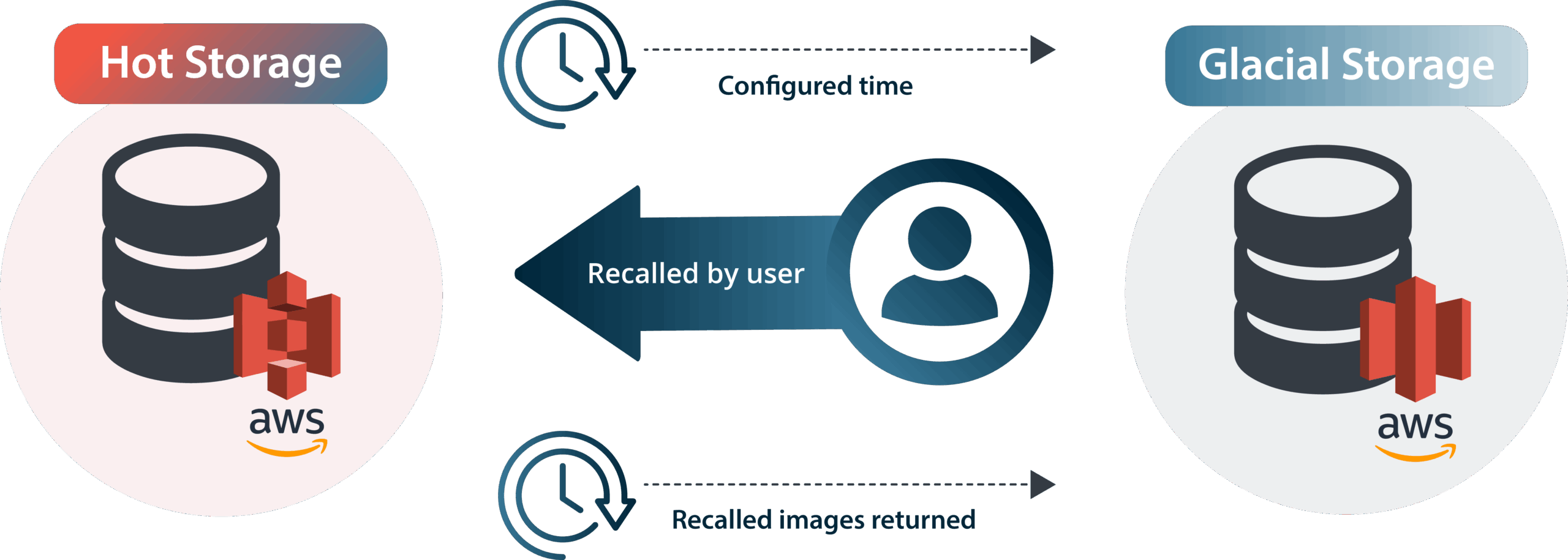
Deploy HALO AP Dx in a Fully Managed Cloud-Hosted Environment
In addition to on-premises deployments, Indica Labs offers fully optimized cloud-hosted HALO AP Dx environments on Amazon Web Services (AWS). Our team handles all aspects of implementation and ongoing maintenance, ensuring a high-performance, scalable, and secure environment with significantly reduced storage costs. You retain full ownership of your AWS account while our AWS Certified Solutions Architects manage both the infrastructure and HALO AP Dx software environment.
Discover the benefits of cloud-hosted deployments in our latest blog post or connect with our Cloud Services team today using the form below to see if a managed cloud deployment is right for your organization.
Want to Learn More?
Fill out the form below to request a recorded or live demo of HALO AP Dx or any of our other products or services.
You can also drop us an email at info@indicalab.com
Products & Services
Interested in purchasing or learning more about our products and services? Our highly trained application scientists are a couple of clicks away.
Software Maintenance & Support Coverage
Interested in purchasing an SMS plan? We would be happy to give you a quote.
Technical Support
Need technical support? Our IT specialists are here to help.
HALO AP Dx (K252762) is FDA-cleared for primary diagnostic use with the Leica Biosystems’ Aperio GT 450 DX scanner and Hamamatsu NanoZoomer® S360MD Slide scanner in the USA. In addition, HALO AP Dx provides built-in compliance with FDA 21 CFR Part 11 and HIPAA.
HALO AP® is CE-IVDR marked for in-vitro diagnostic use in Europe, the UK, and Switzerland. HALO AP® is For Research Use Only in the USA and is not FDA cleared for clinical diagnostic use. In addition, HALO AP® provides built-in compliance with FDA 21 CFR Part 11, HIPAA, and GDPR.
WBS-MAR-000010v2