Indica Labs Sets New Standard for Molecular Testing in Breast Cancer with Expansion of AI Macrodissection Portfolio
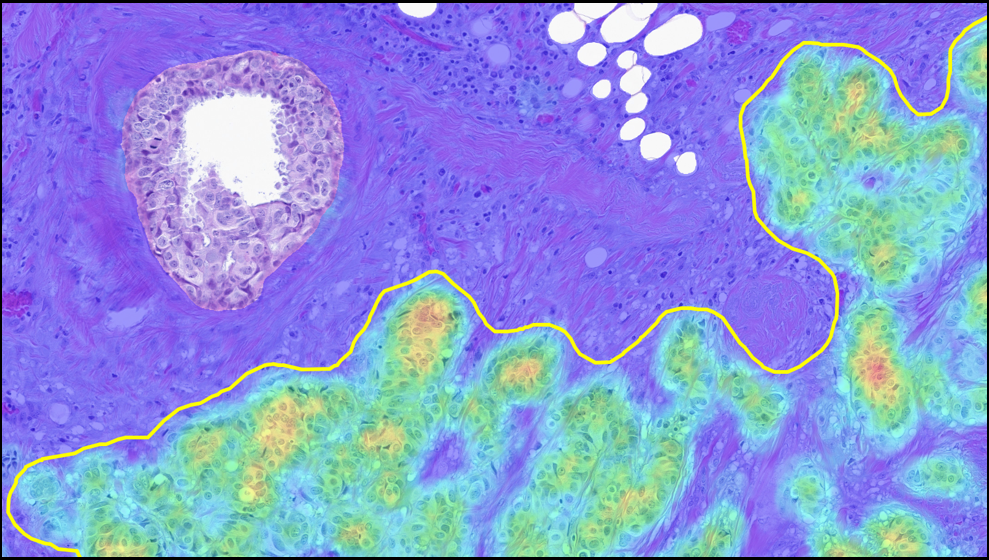
Albuquerque, NM, February 10, 2025 – Indica Labs, the world’s leading provider of AI-powered digital pathology solutions, today announced the expansion of its macrodissection portfolio with the launch of Breast Macrodissect AI . The suite now includes advanced algorithms for non-small cell lung cancer (NSCLC), colorectal cancer (CRC), and breast cancer, with prostate cancer planned for release in early 2026.