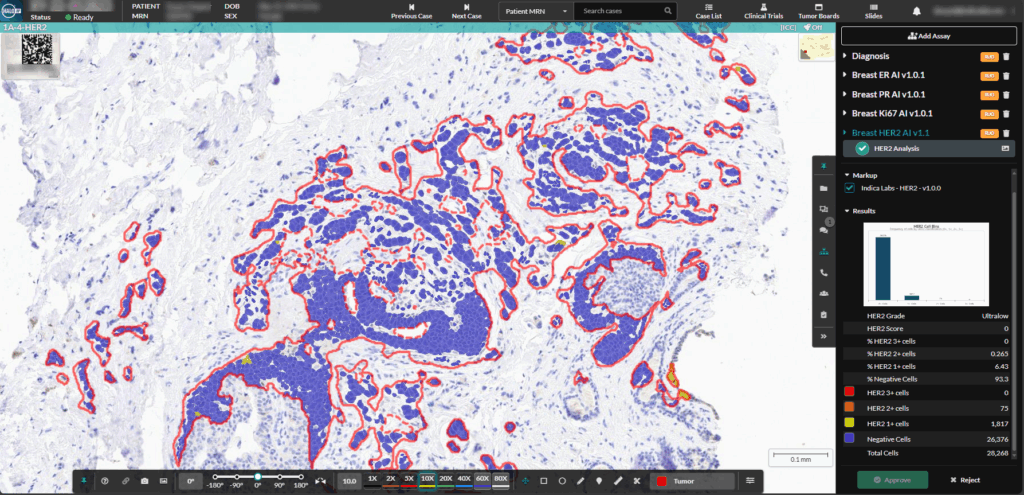
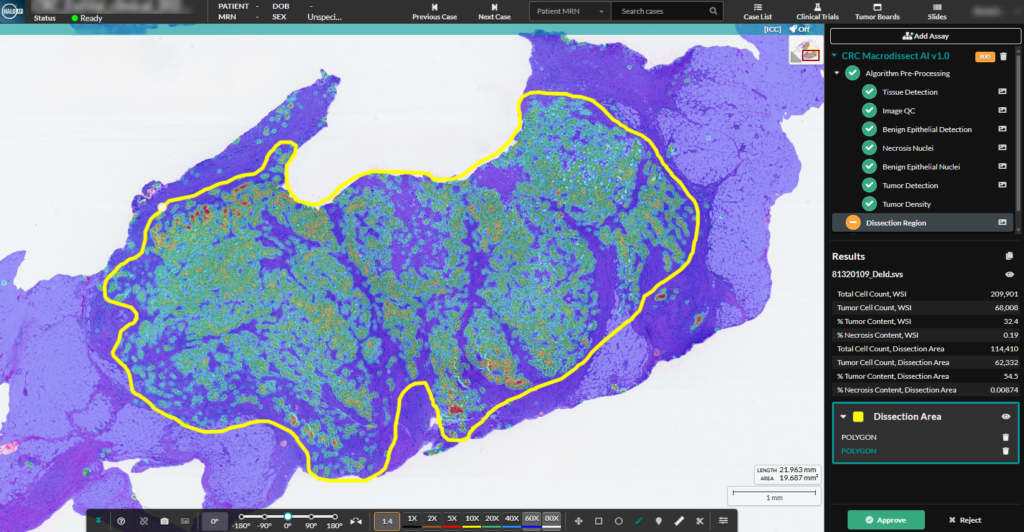
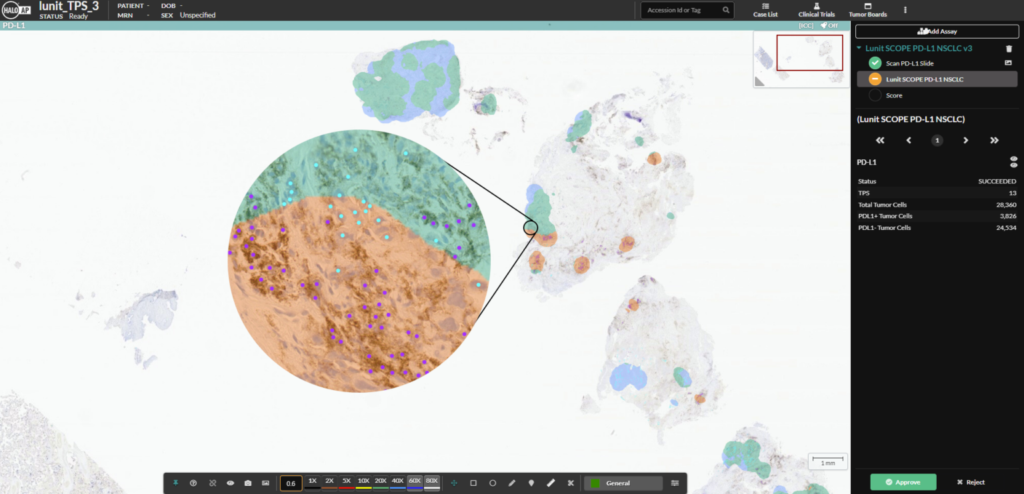
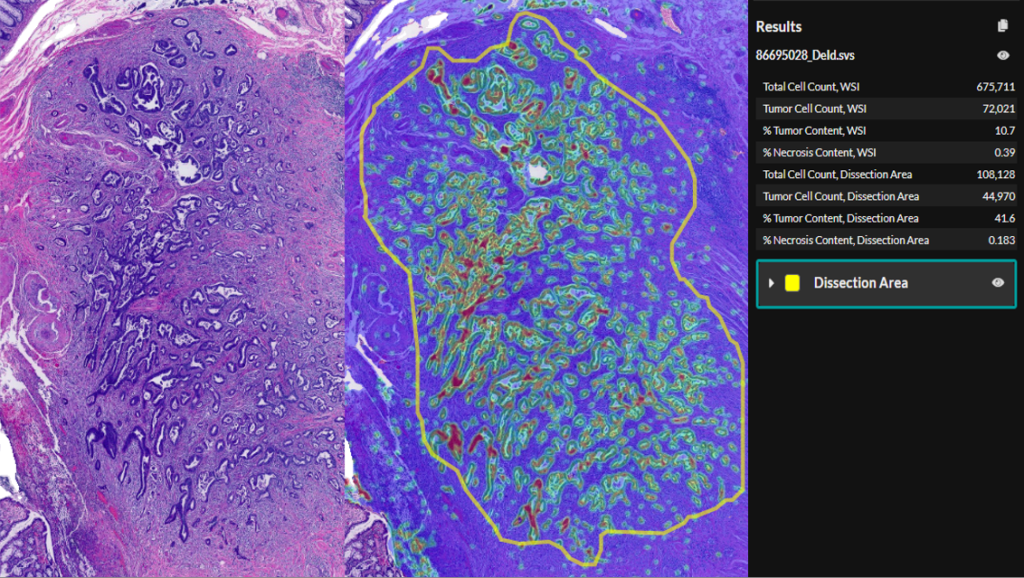
Migrating from Aperio eSlide Manager to Indica Labs HALO Image Management Systems
Migrating from Aperio eSlide Manager to Indica Labs HALO Image Management Systems
Migrating from Aperio eSlide Manager to Indica Labs HALO Image Management Systems Read More »