Unlocking the Potential of Antibody-Drug Conjugates (ADC) with HALO® Image Analysis and Indica Labs Pharma Services

- By Ben Dyer
- October 27, 2025
Antibody-drug conjugates (ADC) represent one of the most promising classes of next-generation cancer therapeutics, combining antibody-based targeting with the potency of chemotherapy. These precisely engineered constructs enable directed delivery of cytotoxic drugs to cancer cells while minimizing the systemic effects of traditional chemotherapy.
Since the first ADC was approved by the US Food and Drug Administration in 2000, over a dozen ADC have been approved globally, offering powerful new treatment options targeting various hematological malignancies and solid tumors.¹ The success of these drugs depends on researchers’ ability to identify appropriate biomarkers, objectively quantify expression at the (sub)cellular level, and understand spatial relationships within the tumor microenvironment (TME). In this blog post, we provide an overview of ADC and share insights from Indica Labs Pharma Services into how AI-powered HALO® image analysis can empower ADC development with accurate, reproducible, and biologically meaningful insights, including metrics generated by quantitative continuous scoring (QCS).
An Overview of ADC
No matter the target, each ADC shares a similar basic design comprising three key components: a monoclonal antibody (mAB) that targets specific cancer-associated antigens, a potent cytotoxic agent, and a chemical linker bridging the two.
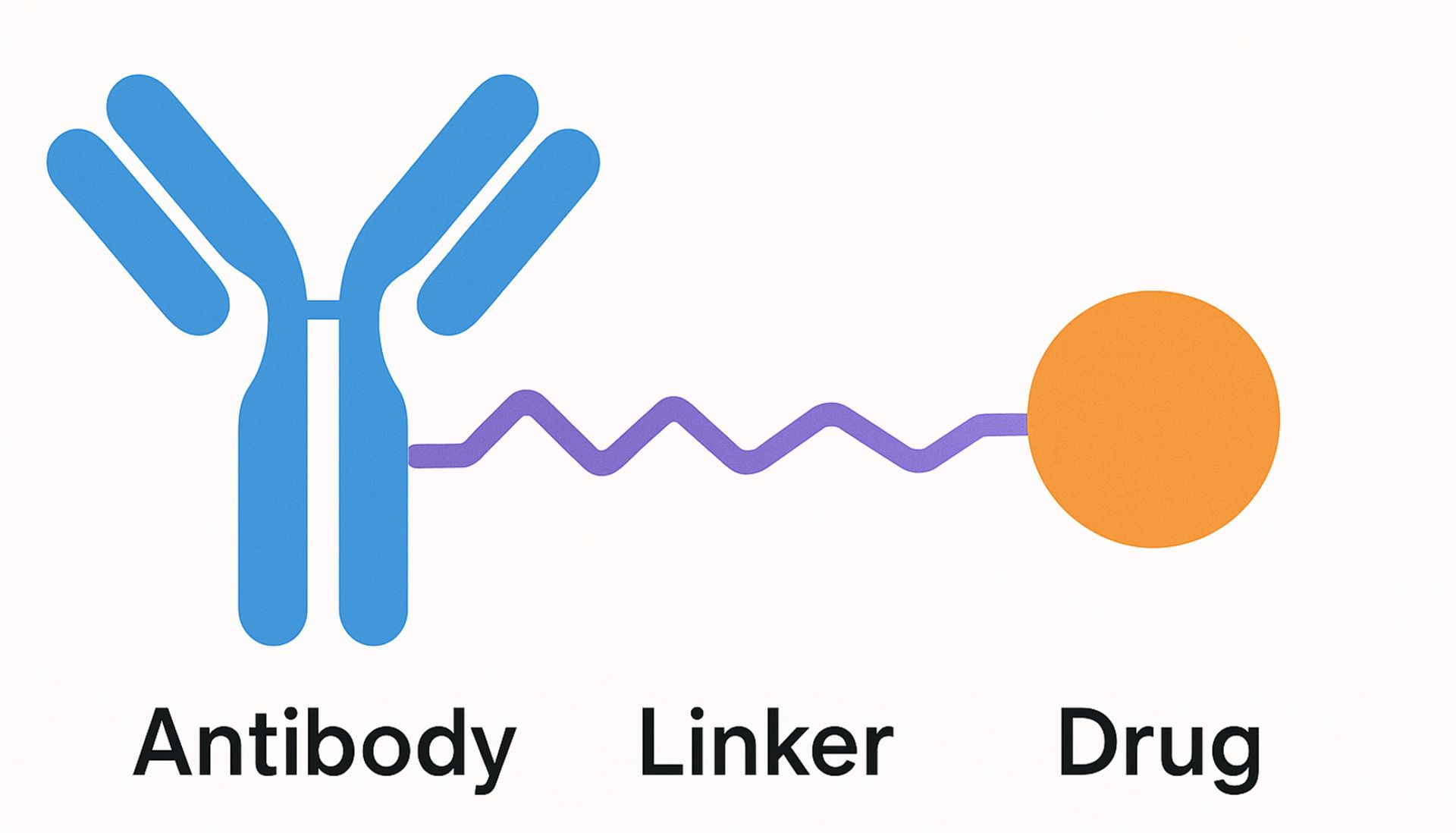
ADC can have multiple, interconnected effects on target and neighboring cells, with the primary mechanism of action beginning with binding to target-expressing cells via the ADC mAB. Following internalization into the cell and transit through the endocytic pathway, the drug component of the ADC is released into the cytoplasm following degradation of the mAB or cleavage of the linker, inducing cell death.
Additionally, cytotoxic components of the ADC may diffuse out of target cells and affect neighboring cells, resulting in a “bystander effect” that can kill target-negative cancer cells and otherwise reshape the TME. Separate from their drug-mediated cytotoxicity, when ADC are present on the surface of target cells their mAB can elicit immune responses including antibody-dependent cellular cytotoxicity, antibody-dependent cellular phagocytosis, and complement-dependent cytotoxicity.¹
The direct connection between the specificity and efficacy of ADC, along with their potential bystander effects and promotion of anti-cancer immunity, underscores the vital importance of extensively characterizing target cells and the TME as part of ADC development. Target antigen expression levels, immune cell types and spatial distribution, the presence of immune-evasive phenotypes, and many other factors fundamentally influence ADC design and efficacy.¹, ²
How Digital Pathology Transforms ADC Development
Studies have shown variability in the clinical response to ADC treatment, highlighting the need for accurate selection of biomarkers that will predict which patients are most likely to benefit from treatment.³ Conventional manual scoring of stained immunohistochemistry slides is subjective, complicated by intra- and inter-observer variability, and by its nature necessitates placing biomarker expression into bins, such as low, medium, and high.⁴
Quantitative digital pathology is the next step in image analysis for ADC development, enhancing standardization and reproducibility while allowing quantitative, continuous measurement of biomarkers on a per-cell basis. Tools such as the HALO® image analysis platform enable unbiased quantitative assessment of biomarker expression across cellular compartments and of spatial relationships between cells. These insights provide researchers with a richer data set as they optimize ADC for specific target expression profiles and TME and characterize patient responses.²
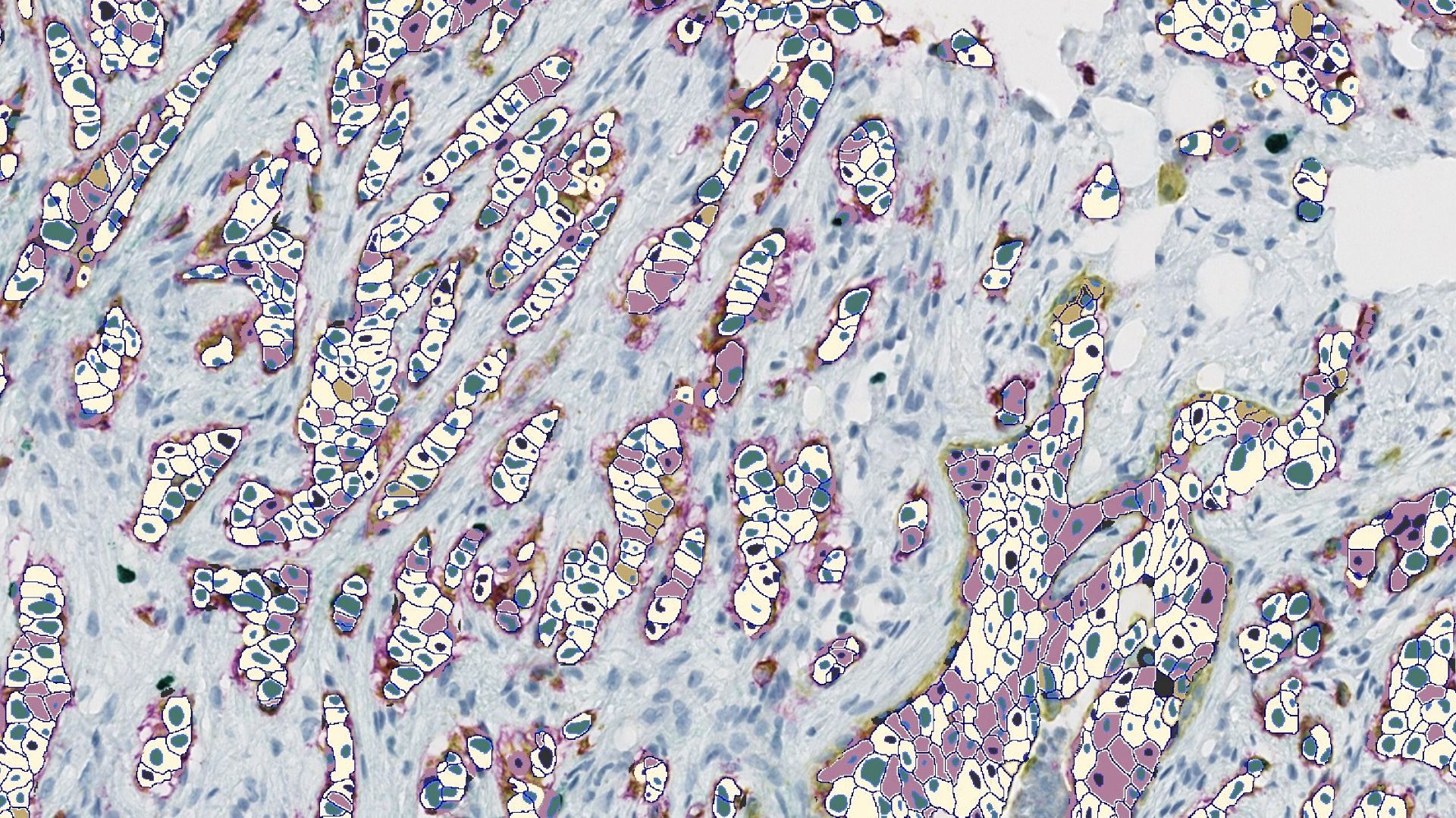
Leveraging HALO for ADC Research
Indica Labs’ Pharma Services team leverages HALO and the HALO AI deep learning toolkit to empower customers’ ADC development projects with accurate, in-depth image analysis, from tissue segmentation to advanced spatial analysis. The team has experience with diverse ADC projects, and while the specifics of each project differ, the foundational image analysis workflow typically includes the following steps:
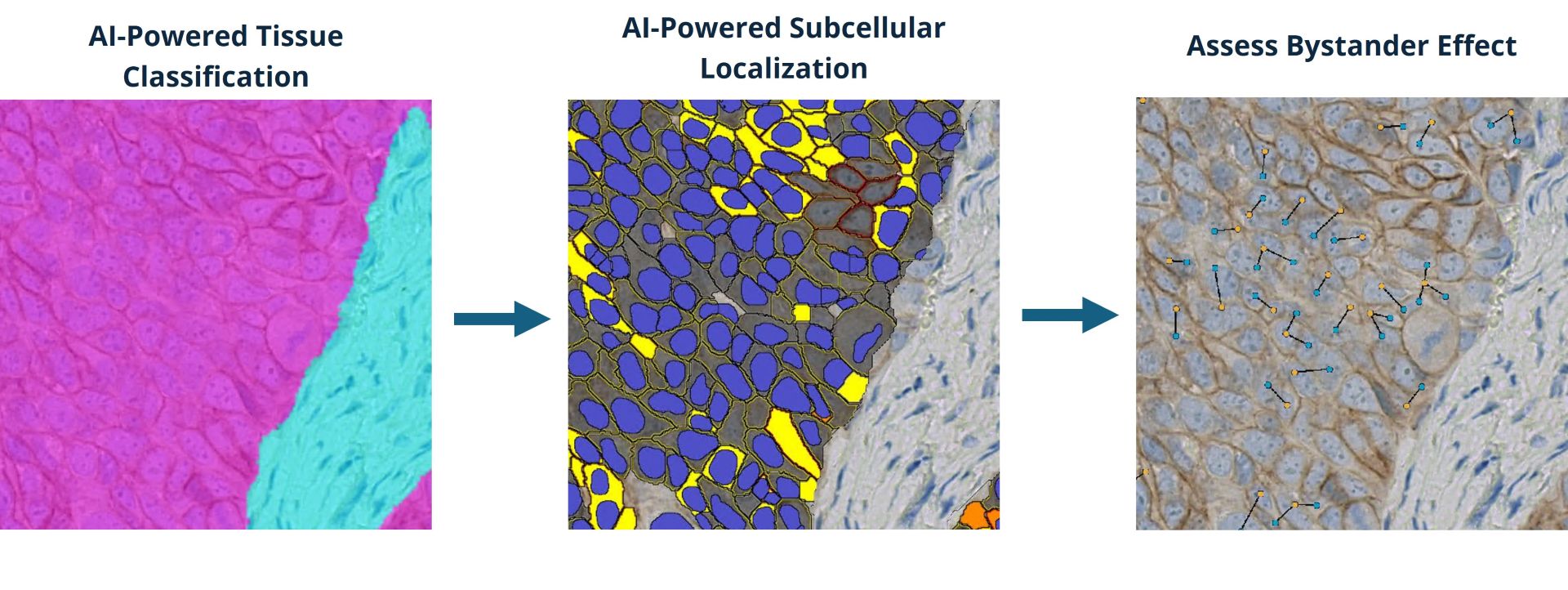
- Tissue classification: Tumor and stroma or other classes of interest are delineated using deep-learning networks developed using HALO AI.
- Nuclear and membrane segmentation: Additional HALO AI networks power nuclear and membrane segmentation, enabling accurate definition of ADC-positive and ADC-negative cell populations and analysis of biomarker distribution in cells.
- Biomarker quantification: Target expression is assessed by QCS, in which biomarker expression is quantified as mean optical density in the segmented cell nuclei, cytoplasm, and membrane. QCS enables spatial analysis of biomarker location in cells, with distribution of some targets, such as TROP2, having been shown to be predictive of clinical outcomes.⁵ HALO has offered per-cell, compartment-level intensity data for over a decade, and the Pharma Services team has extensive experience leveraging this objective and continuous data.
- Bystander effect evaluation: The HALO Spatial Analysis module is used to measure the proximity of target-negative cells to target-positive cells and the invasion of immune cells into the TME.
Together, the steps offer a streamlined workflow that produces a wealth of data on ADC target expression on a cell-by-cell level with subcellular resolution, as well as rich phenotypic and spatial data on other cells in the TME. This data enables Pharma Services’ customers to more accurately characterize ADC targets, optimize dosing strategies, and validate candidate therapies.
Partnering with Pharma Services
Developing ADC is complex, requiring expertise in oncology, immunology, and advanced image analysis. The members of Indica Labs Pharma Services are image analysis experts with backgrounds in digital pathology, immuno-oncology, cancer biology, neuroscience, bioinformatics, and data science. The team works with customers to tailor analysis workflows to project-specific needs and collaborates closely with pathologists to ensure analyses are both biologically accurate and clinically meaningful.
From biomarker discovery to spatial characterization of the bystander effect, our team leverages the latest tools in digital pathology and optimized workflows to deliver representative, high-quality data. With a proven track record of supporting ADC programs, Pharma Services provides the scientific and technical image analysis expertise needed to help drive the development of these cutting-edge therapies. In addition, the partnership between Indica Labs and Leica Biosystems offers customers the synergized expertise and resources of both companies, helping pharma teams accelerate the development of the next generation of precision diagnostics.
Follow the link below to reach out to the Indica Labs Pharma Services team and discuss how they can help advance your ADC project. Want to learn more about the team and their processes? Check out our prior introductory, QC process, and AI classifier development blog posts.
Get Started with Pharma Services
References
1. Wang R, Hu B, Pan Z et al. Antibody–Drug Conjugates (ADCs): current and future biopharmaceuticals. Journal of Hematology & Oncology. 18, 51 (2025). DOI: 10.1186/s13045-025-01704-3.
2. Kapil A, Spitzmüller A, Brieu N et al. HER2 quantitative continuous scoring for accurate patient selection in HER2 negative trastuzumab deruxtecan treated breast cancer. Scientific Reports. 14, 12129 (2024). DOI: 10.1038/s41598-024-61957-9.
3. Liu K, Li M, Li Y et al. A review of the clinical efficacy of FDA-approved antibody‒drug conjugates in human cancers. Molecular Cancer. 23, 62 (2024). DOI: 10.1186/s12943-024-01963-7.
4. Rizzardi A, Johnson A, Vogel R et al. Quantitative comparison of immunohistochemical staining measured by digital image analysis versus pathologist visual scoring. Diagnostic Pathology. 7, 42 (2012). DOI: 10.1186/1746-1596-7-42.
5. Garassino M, Sands J, Paz-Ares L et al. PL02.11 Normalized Membrane Ratio of TROP2 by Quantitative Continuous Scoring is Predictive of Clinical Outcomes in TROPION-Lung 01. Journal of Thoracic Oncology. 19(10), S2-S3 (2024). DOI: 10.1016/j.jtho.2024.09.015.