KLAS Recognition as a Catalyst for What Comes Next
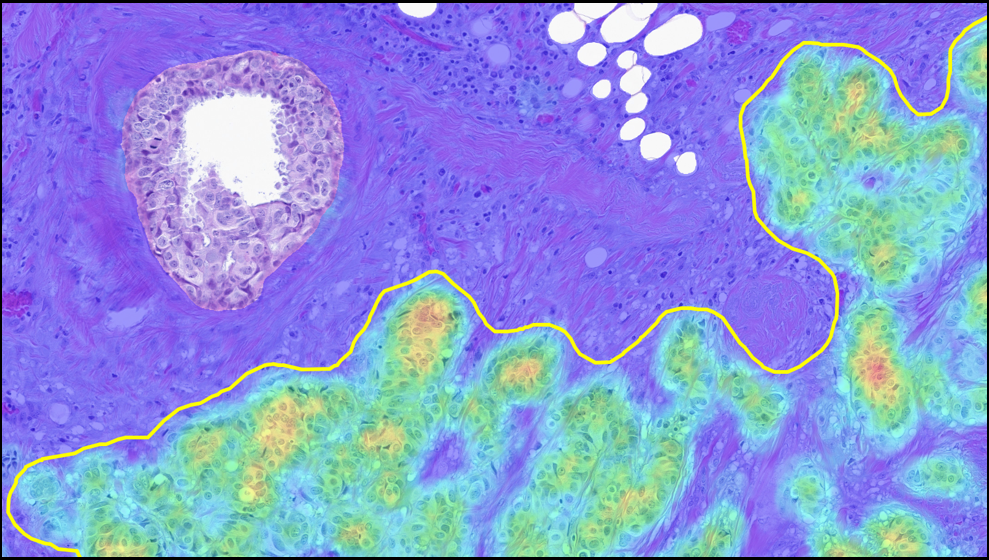
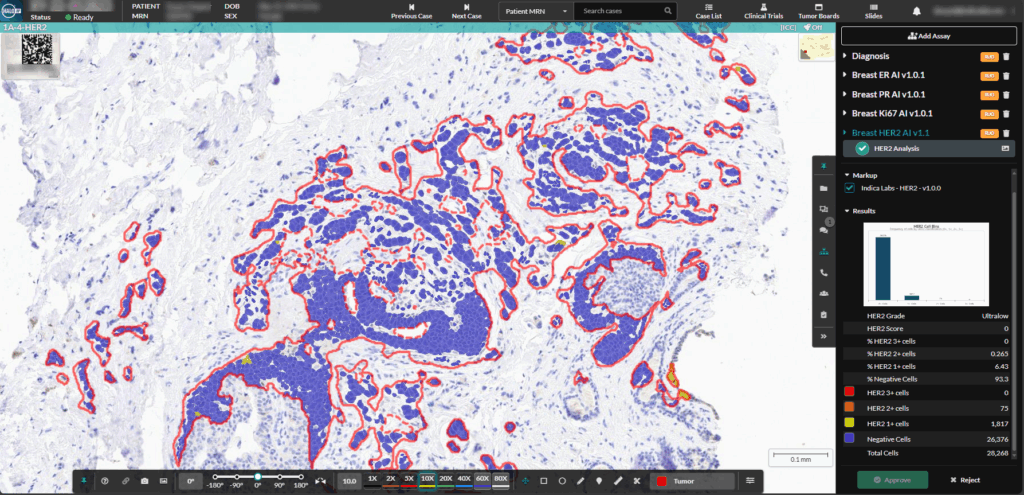
We are proud to mark a significant milestone: HALO AP® earned the highest overall customer rating in the 2026 KLAS Global Software report for digital pathology. Because KLAS distinction is driven entirely by direct customer feedback, this recognition reflects the real-world performance, reliability, and impact of our platform in daily pathology practice.
KLAS Recognition as a Catalyst for What Comes Next Read More »